Tungsten pentaboride, WB5-x, has recently gained attention as a potential catalyst due to its unique properties. A group of researchers led by Professor Alexander Kvashnin has conducted a study on the stable surfaces of the WB5-x crystal and discovered that it offers several advantages over traditional catalysts. This article delves into the findings of the study and explores the potential applications of tungsten pentaboride as a catalyst in various industries.
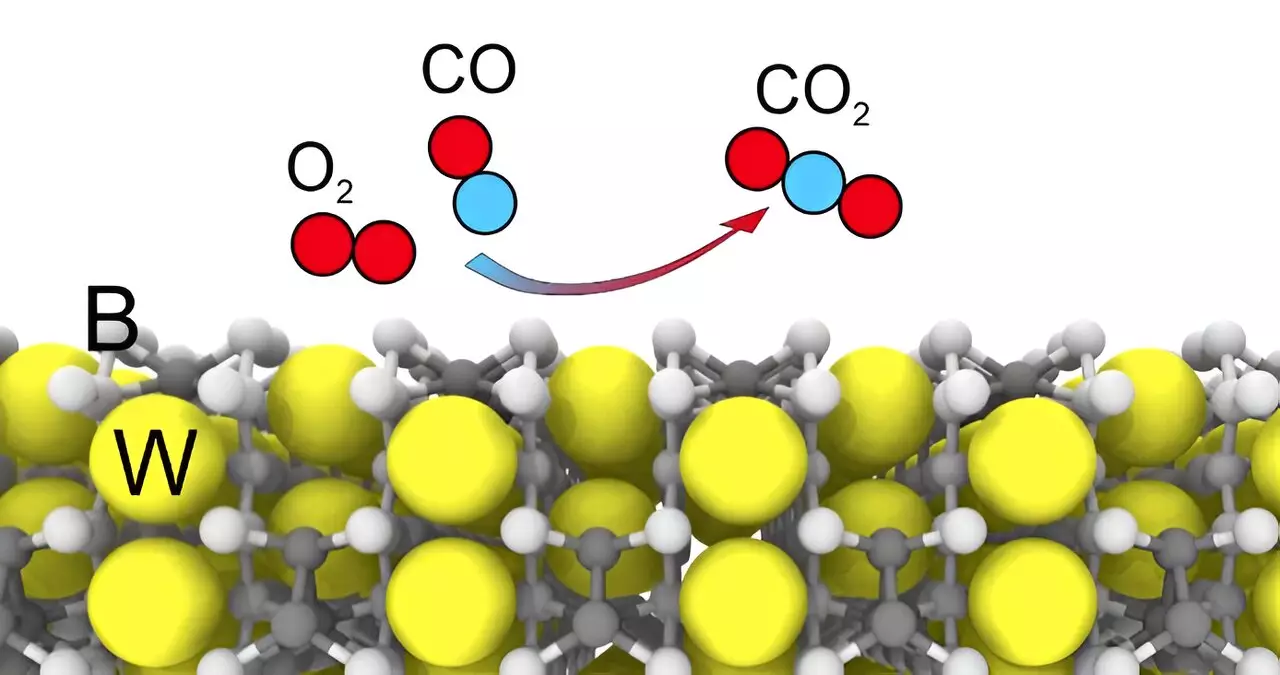
One of the key findings of the research is that tungsten pentaboride is not poisoned by sulfur-containing impurities, making it highly stable and long-lasting as a catalyst. This sets it apart from catalysts based on noble and rare earth metals, which are susceptible to poisoning from such compounds. The researchers found that the compound’s catalytic properties are enhanced by the presence of boron, with higher concentrations of boron leading to improved performance. This discovery challenges the conventional wisdom that metal atoms are the primary active centers of catalysts, highlighting the importance of boron in catalytic processes.
The implications of these findings are significant for various industries that rely on catalysts for key processes. Tungsten pentaboride could be used as a catalyst or co-catalyst in filters for cleaning industrial exhaust gases, mining precious metals, photocatalytic production of hydrogen, and other applications. Its resistance to poisoning from sulfur-containing compounds makes it an attractive option for industries where catalyst longevity is a crucial factor. For example, in the production of methane from carbon dioxide or hydrogen from an aqueous solution of ethanol, tungsten pentaboride could significantly enhance reaction efficiency.
The study also identified stable surfaces of the tungsten pentaboride compound, with one surface predominantly consisting of boron and the other of tungsten atoms. The researchers observed that boron plays an active role in both adsorption and catalysis processes, underscoring its importance in the compound’s catalytic activity. By comparing the two surfaces, the team was able to gain insights into how boron influences the catalytic properties of tungsten pentaboride. This in-depth analysis sheds light on the mechanisms underlying the compound’s superior performance as a catalyst.
The research conducted by Professor Alexander Kvashnin and his team has highlighted the potential of tungsten pentaboride as a catalyst with unique advantages. Its resistance to poisoning from sulfur-containing compounds, high catalytic activity, and stable surfaces make it a promising candidate for various industrial applications. Further studies and experiments could explore the full range of possibilities offered by tungsten pentaboride as a catalyst, opening up new avenues for innovation in catalysis.

Leave a Reply