Since chemist Sir Arthur Michael first reported nucleophilic addition reactions to the β-position of α,β-unsaturated carbonyl compounds in 1887, these reactions, commonly known as Michael addition reactions, have been extensively researched. However, achieving anti-Michael addition reactions, which involve nucleophilic addition to the α-position, has proven to be challenging. This difficulty arises from the higher electrophilicity of the β-position compared to the α-position, making it more challenging to carry out reactions in the opposite direction.
Historically, researchers have attempted to overcome the obstacles associated with anti-Michael addition reactions through two main methods. The first involves restricting the addition position through intramolecular reactions. The second method includes introducing a strong electron-withdrawing group at the β-position. While these approaches have been explored, they are not considered ideal for synthesizing complex molecules via the anti-Michael reaction.
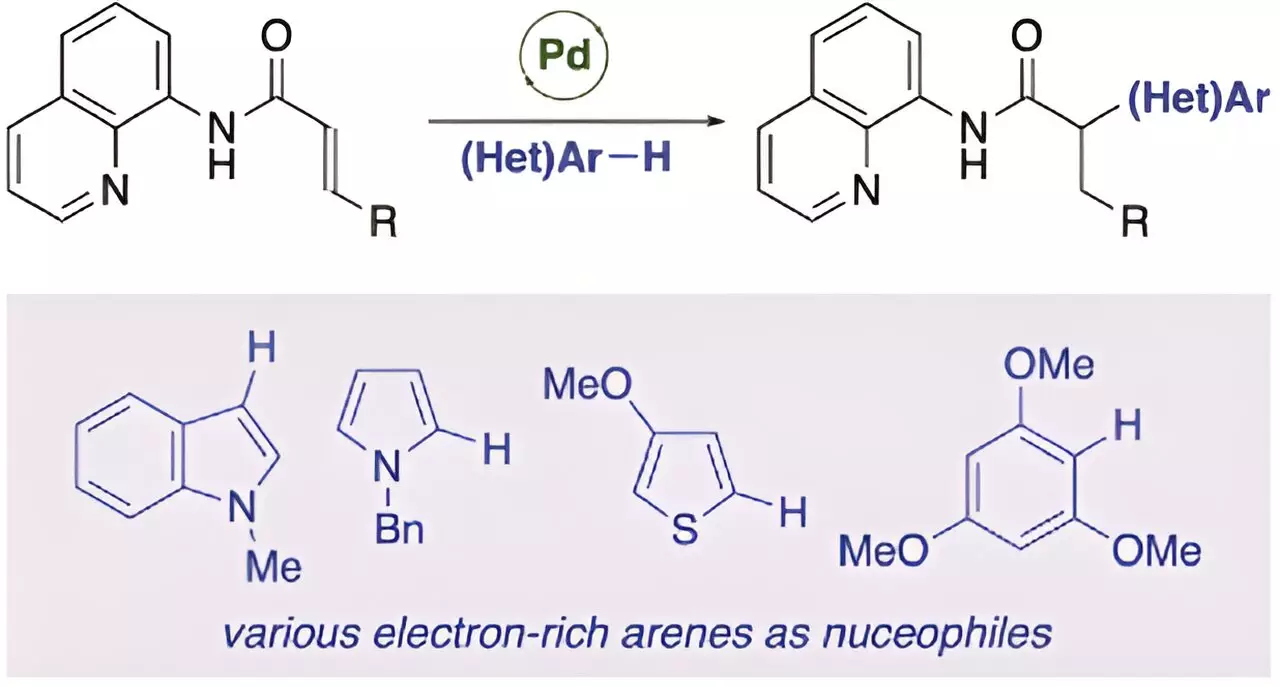
Recently, a global team of researchers, led by Professor Takanori Matsuda from the Department of Applied Chemistry at Tokyo University of Science in Japan, achieved a breakthrough in this area. The team, which also included Ryota Moro and Assistant Professor Hirotsugu Suzuki, successfully conducted a palladium-catalyzed anti-Michael addition reaction of acrylamides, marking the first example of an anti-Michael-type addition reaction.
The researchers discovered that the presence of a catalytic amount of palladium(II) trifluoroacetate (Pd(TFA)2) could facilitate the anti-Michael addition of indole to acrylamide with an aminoquinoline group as a directing group. This resulted in the production of the addition product in high yield. The study sheds light on the potential of using directing groups to stabilize reaction intermediates and facilitate anti-Michael type addition reactions.
The implications of this research are significant, particularly in the synthesis of α-substituted carbonyl compounds, which are widely used in pharmaceuticals. The anti-Michael type addition reaction offers a one-step process with high atomic efficiency, making it a valuable tool in organic synthesis. The ability to efficiently produce α-substituted carbonyl compounds through this method has the potential to revolutionize pharmaceutical development and other areas of organic chemistry.
Overall, the groundbreaking research by Professor Matsuda and his team represents a significant advancement in the field of anti-Michael addition reactions. By utilizing palladium-catalyzed reactions and introducing directing groups, the researchers have opened up new possibilities for synthesizing complex molecules efficiently. The study not only expands our understanding of organic reactions but also paves the way for the development of novel pharmaceuticals and other valuable compounds.

Leave a Reply