Understanding the interactions between phosphorous acid and platinum catalysts in high-temperature PEM fuel cells is crucial for enhancing their efficiency and longevity. Recent experiments conducted at BESSY II using tender X-rays have revealed a more intricate picture of the multiple oxidation processes taking place at the platinum-electrolyte interface. These findings challenge previous assumptions and shed light on the potential impact of humidity variations on the performance of fuel cells.
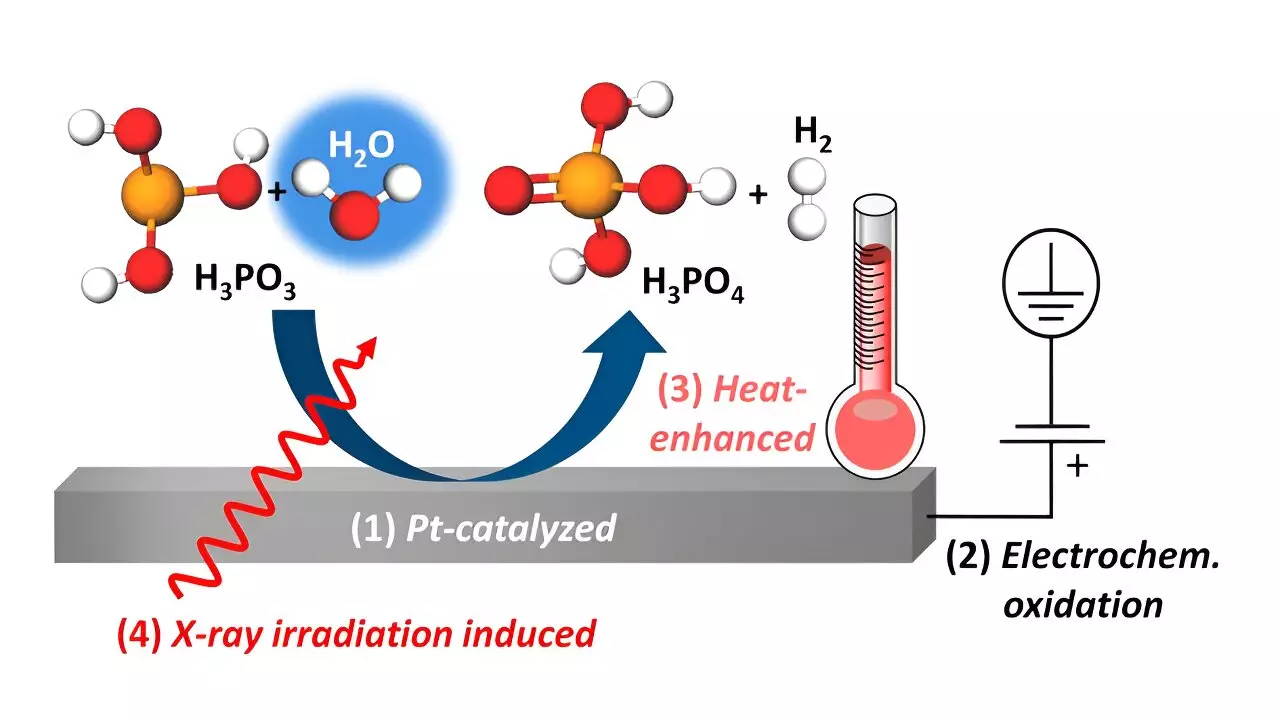
High-temperature polymer electrolyte membrane fuel cells (HT-PEMFCs) are considered promising for micro-stationary electricity sources due to their ability to convert chemical energy from hydrogen into electrical energy. However, one of the drawbacks of these fuel cells is the leaching of phosphoric acid (H3PO4) proton conductor from the H3PO4-doped polybenzimidazole membrane, which can poison the platinum catalyst. Recent studies have also highlighted the possibility of H3PO4 being reduced to H3PO3 during operation, further contributing to catalyst poisoning and a decrease in fuel cell performance.
Insights from In-Situ Studies
Prof. Dr. Marcus Bär’s research team has uncovered the complex interactions between platinum catalysts and aqueous H3PO3/H3PO4 at the platinum-electrolyte interface. Through in-house designed heatable electrochemical cells and in situ tender X-ray studies at the OÅSE end-station, the team has demonstrated different oxidation pathways for H3PO3, including platinum-catalyzed chemical oxidation, electrochemical oxidation under positive potential bias, and heat-promoted oxidation. These results have been validated through ion-exchange chromatography and in situ electrochemical characterizations, providing a comprehensive understanding of the oxidation behavior of H3PO3.
An interesting finding from the study is the significant influence of humidity on the oxidation processes involving water. The results suggest that controlling the humidification within HT-PEMFCs could potentially oxidize H3PO3 back to H3PO4, thereby preventing catalyst poisoning and improving the overall efficiency of fuel cells. These insights pave the way for fine-tuning the operating conditions of HT-PEM fuel cells to mitigate degradation pathways and enhance their performance.
Implications for Future Energy Supply
The work conducted by Prof. Dr. Marcus Bär’s team not only clarifies a key degradation pathway of fuel cells but also offers valuable contributions towards establishing an H2-based energy supply. By unraveling the complexities of phosphorous acid-platinum catalyst interactions and highlighting the role of humidity in oxidation processes, this research sets the stage for optimizing the design and operation of fuel cells for sustainable energy production.

Leave a Reply