Peptides are becoming increasingly recognized as vital therapeutic agents for addressing various medical conditions. Unlike small-molecule drugs, peptides have the ability to target complex biological processes in a more precise manner. They are also generally less complex and more cost-effective than larger biological drugs such as antibodies. Over 100 FDA-approved peptide drugs have been introduced to the market since the development of the first peptide hormone insulin in 1923. Among these drugs, approximately 40 of them, used for treating a wide range of diseases including cancer, cardiovascular issues, and metabolic disorders, contain at least one tryptophan (Trp) residue, which plays a crucial role as a key amino acid in these molecules.
Although the modification of Trp residues in peptide molecules can have a significant impact on drug-target interactions and enhance properties such as drug stability, bioavailability, and pharmacokinetics, it is not without its challenges. These challenges include the necessity for high levels of selectivity, such as chemoselectivity, regioselectivity, and stereoselectivity, when making transformations to these functionally dense molecules. Peptides also possess nucleophilic functionalities that make them sensitive to redox conditions, which can complicate modification processes. Furthermore, the limited solvents available for dissolving unprotected peptides can pose additional obstacles. As a result, developing site-specific late-stage modifications to peptides has proven to be a daunting task for researchers in the field of drug development.
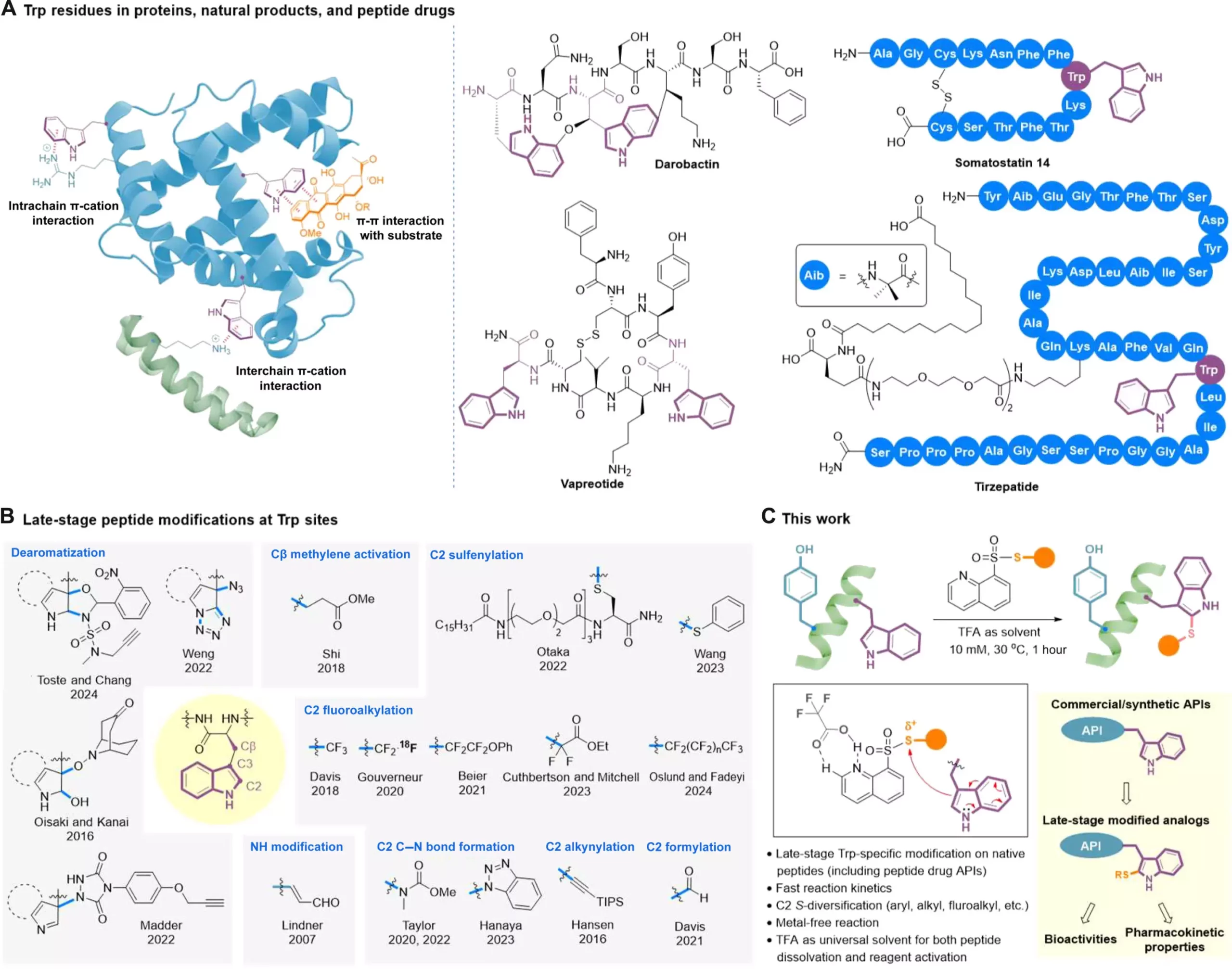
A recent breakthrough in peptide modification techniques comes from a team led by Professor Xuechen Li from the Department of Chemistry at The University of Hong Kong. The team has developed a clickable tryptophan modification strategy that allows for the customization of specific parts of a peptide molecule, even during the late stages of drug development. This innovative late-stage diversification technique enables researchers to fine-tune peptides after the core structure has already been established. The strategy involves a late-stage catalyst-free C2-sulfenylation reaction using S-modified quinoline-containing thiosulfonate reagents.
Application and Impact of the New Method
Through this groundbreaking method, researchers can efficiently introduce a variety of functional groups onto tryptophan (Trp) residues within native peptide structures. These groups include trifluoromethylthio, difluoromethylthio, (ethoxycarbonyl) difluoromethylthio, alkylthio, and arylthiol. The use of trifluoroacetic acid (TFA) as the optimal solvent plays a critical role in activating the reagents through hydrogen bond interaction. Additionally, TFA’s superior dissolving capability for hydrophobic and aggregation-prone peptides ensures the method’s applicability to challenging molecules such as lipopeptides and self-assembling peptides, even at high concentrations. This method has already been successfully applied to the late-stage modification of several on-market peptide drugs and bioactive glycopeptides, showcasing its potential for diversifying peptide-based active pharmaceutical ingredients.
The team led by Professor Li believes that this single-step clickable late-stage Trp modification method will serve as a robust platform for generating structural analogs in a cost-efficient manner. It can meet the demands for optimizing drug activities and pharmacokinetic properties, making it a valuable tool for medicinal chemists, peptide chemists, and chemical biologists. The method’s versatility makes it applicable not only to existing peptide drugs but also to natural products and potential drug leads identified through various screening processes. Ultimately, this innovative peptide modification technique has the potential to revolutionize drug development and open up new possibilities for creating novel therapeutic agents.

Leave a Reply