The immune system plays a crucial role in protecting the body against pathogens and abnormal cells, such as cancer cells. Antigen processing is a complex mechanism by which diseased cells present antigens on their surface to alert the immune system. A recent study published in Angewandte Chemie International Edition has introduced a groundbreaking technique that allows for real-time analysis of antigen processing using light-triggered antigen release.
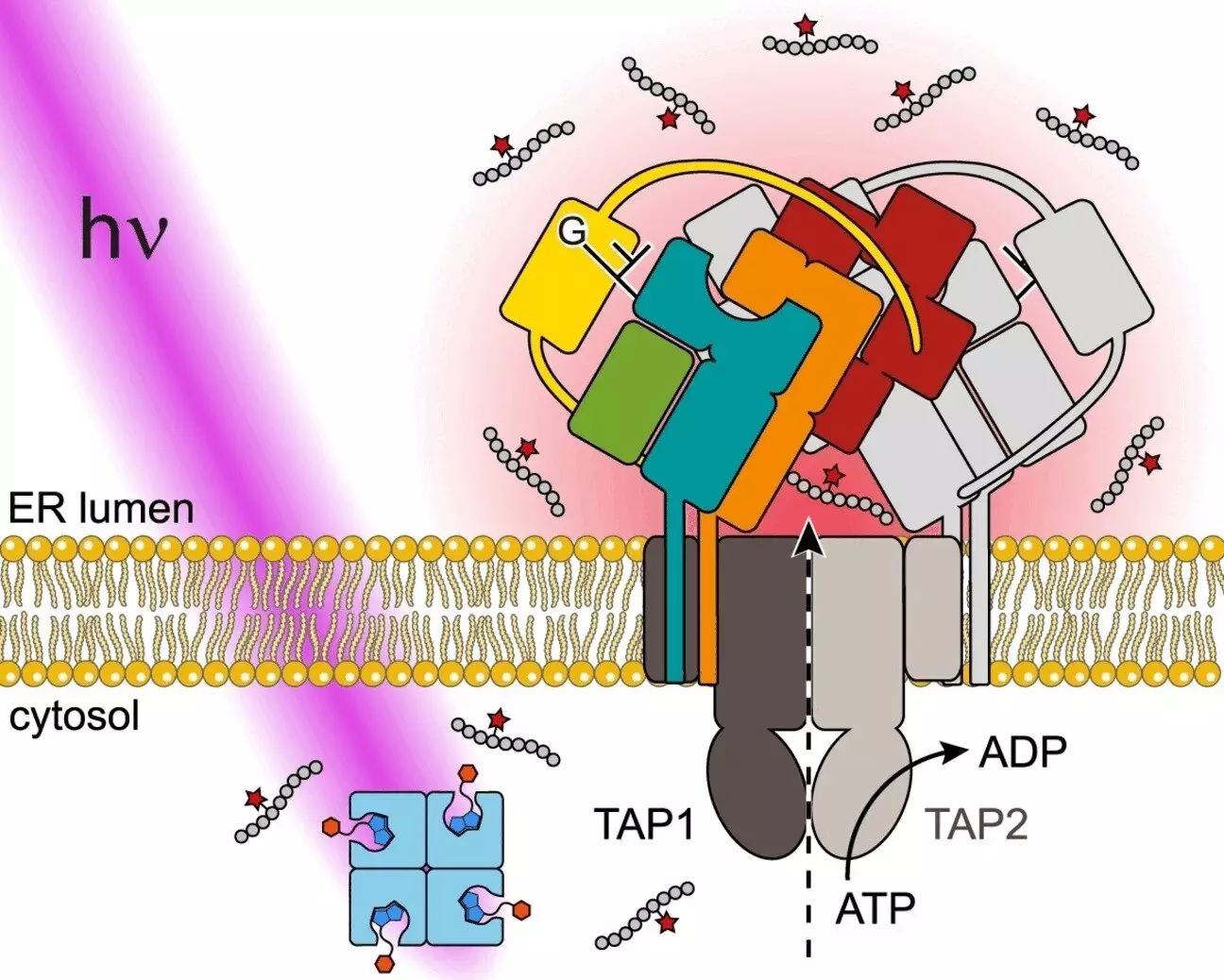
In our cells, both endogenous and foreign proteins are broken down into small peptides and transported to the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP). The peptides are then loaded onto major histocompatibility complex class I (MHC I) molecules by the peptide loading complex (PLC) for presentation on the cell surface. This process is essential for immune surveillance and response to foreign invaders.
A team of researchers from the University of Frankfurt in Germany, led by Ralph Wieneke and Robert Tampé, have developed a photostimulated antigen release system to study antigen flux in real time. This system involves trapping antigens in a “cage” that can be opened by light, resulting in the immediate release of antigens at a specific location and time. This method allows for precise control over the antigen release process, enabling researchers to study antigen processing dynamics.
One of the key advantages of using light stimulation for antigen release is the ability to control the timing and location of antigen presentation. Light can be precisely dosed and is noninvasive, making it suitable for experiments in living cells. By utilizing a peptide derived from an HIV antigen as a model, the researchers were able to demonstrate the versatility and effectiveness of this novel approach in studying antigen transport dynamics.
This new antigen release system has the potential to revolutionize the study of antigen processing and immune surveillance. By gaining insights into the mechanistic principles of antigen translocation and PLC assembly, researchers can better understand the quality control mechanisms of peptide-MHC complexes. Furthermore, this system can be used to trace antigen transport in various disease models, such as human lymphoma cell lines.
The development of a light-triggered antigen release system represents a significant advancement in the field of antigen processing research. This innovative approach provides researchers with a powerful tool to study the dynamics of antigen presentation and immune response in real time. By shedding light on the intricate mechanisms underlying antigen processing, this system holds great promise for future advancements in disease research and therapeutic interventions.

Leave a Reply