Cement is indispensable for modern infrastructure, but the industry is also one of the primary contributors to greenhouse gas emissions, particularly carbon dioxide (CO2). The urgency of climate change has sparked innovations in construction materials and techniques, with carbonation processes emerging as a beacon of hope. Carbonation refers to the method of capturing and converting CO2 into stable minerals within cement-based materials, potentially allowing the construction sector to play a constructive role in combating climate change. This article will explore recent advancements in understanding carbonation mechanisms, highlighting critical research contributions that inform future sustainable practices.
The rising atmospheric levels of CO2 are intimately linked with global warming, prompting scientists and engineers to seek effective methods for carbon capture. Among these methods, the carbonation of cement holds immense promise. When CO2 is dissolved in water, it reacts with materials such as calcium silicate hydrate (C–S–H), a key component of cement, leading to the formation of calcium carbonate precipitates. However, while the basic process is documented, the intricate mechanisms governing carbonation in cement paste remain elusive. Factors such as relative humidity, CO2 solubility, and the calcium-to-silicate (Ca/Si) ratio can significantly influence this process, presenting challenges and opportunities for researchers.
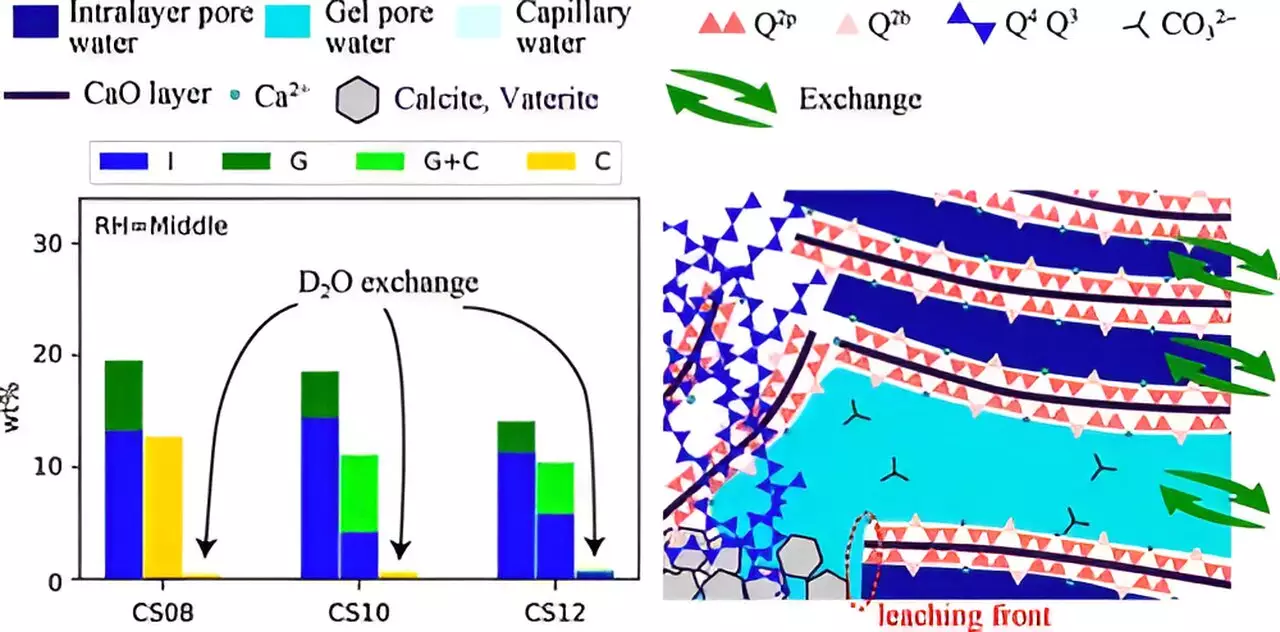
A team led by Associate Professor Takahiro Ohkubo from Chiba University has embarked on groundbreaking research aimed at demystifying the carbonation process. Understanding the structural changes and water transport dynamics within C–S–H under various conditions is vital for optimizing the efficiency of CO2 capture in cement. By employing advanced techniques like 29Si Nuclear Magnetic Resonance (NMR) and 1H NMR relaxometry, the research team is breaking new ground in the study of water’s role in the carbonation process. This innovative approach allows for a deeper examination of how water molecules move through the nanometer-sized pores of C–S–H layers, enhancing the understanding of carbonation kinetics.
Accelerating the Carbonation Process
Natural carbonation of cement takes decades, rendering it impractical to study in laboratory settings. To overcome this limitation, Professor Ohkubo’s team utilized accelerated carbonation processes with elevated CO2 levels—approximately 100% CO2—far exceeding atmospheric concentrations. This laboratory simulation facilitates a controlled environment for observing how variables such as relative humidity and Ca/Si ratios influence carbonation efficiency. The researchers synthesized C–S–H samples under diverse conditions to confirm their findings, collecting data that reveal crucial insights into the carbonation mechanism.
Key Findings and Their Implications
The findings of this research are significant, revealing that carbonation reactions can vary dramatically based on specific conditions. The study’s results demonstrate that both the water transport and structural modifications within C–S–H are critical to understanding how well CO2 can be captured and converted. Notably, it was observed that lower relative humidity and higher Ca/Si ratios led to smaller pore sizes, which effectively reduced the leaching of essential calcium ions. This finding underscores the importance of manipulating these variables when designing new cement compositions that can optimize carbonation rates.
In addition to potential applications in building materials, this research contributes to broader ecological benefits. The techniques and knowledge developed could extend beyond cement, informing our understanding of organic materials’ carbon sequestration in natural environments. The systemic approach employed by the researchers indicates promising pathways toward developing construction materials that not only reduce emissions but actively absorb CO2 from the atmosphere.
The Path Forward
As the construction industry grapples with its environmental impact, the implications of this research present an exciting opportunity for integrating sustainability into building practices. The dual benefits of better material performance and enhanced carbon capture can potentially transform concrete from a liability into an ally in the fight against climate change. As the team led by Professor Ohkubo continues to refine their findings and explore scalable applications, a future where cement actively contributes to CO2 reduction seems increasingly plausible.
Understanding carbonation processes in cement-based materials is a critical step toward tackling climate change. The innovative research by Ohkubo and his collaborators not only elucidates these mechanisms but also propels us toward developing sustainable building solutions capable of both meeting societal needs and safeguarding the planet for future generations.

Leave a Reply