The utilization of CO2 as a chemical raw material has the potential to revolutionize the way we produce essential products while simultaneously reducing emissions and our dependence on fossil fuels. A recent study published in Angewandte Chemie International Edition introduces a groundbreaking metal-free organic framework that could enable the electrocatalytic production of ethylene from CO2, a key chemical building block with widespread applications in industry.
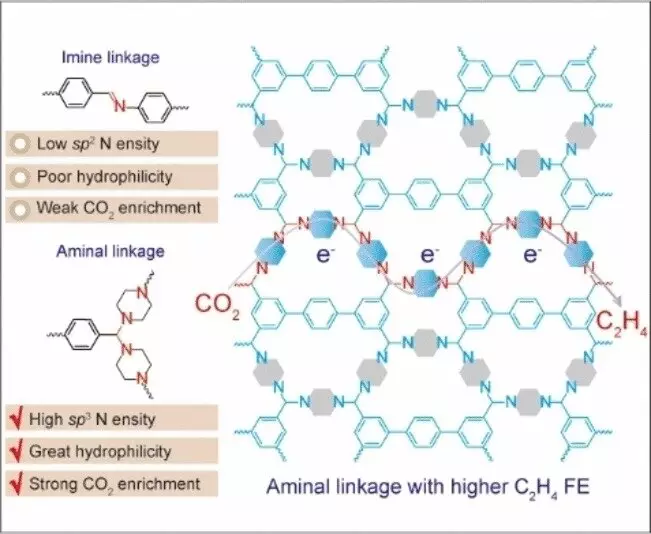
One of the key findings of the study is the critical role played by nitrogen atoms with a specific electron configuration in the catalyst. These nitrogen centers, with a sp3 hybridization, serve as active sites for the electrochemical conversion of CO2 to ethylene. Unlike traditional copper catalysts, this metal-free approach offers advantages in terms of cost and environmental impact, making it a promising alternative for sustainable chemical production.
Metal-free electrocatalysis using nitrogen-containing covalent organic frameworks (COFs) represents a significant advancement in the field of CO2 utilization. COFs are unique in that they are purely organic, porous materials with controllable properties, making them an ideal platform for catalytic reactions. By leveraging the special electron configuration of sp3 nitrogen centers, the researchers were able to achieve high selectivity and performance in the production of ethylene, with a Faraday efficiency of up to 19.1%.
The electrochemical conversion of CO2 to ethylene presents a number of challenges, primarily due to the stable nature of CO2 and the requirement for forming a bond between two carbon atoms. The development of metal-free catalysts like the one described in the study opens up new possibilities for sustainable chemical production. By optimizing the structure of COFs and fine-tuning the interaction between nitrogen centers and CO2 molecules, researchers can continue to improve the efficiency and selectivity of ethylene production.
Implications for Sustainability
The successful synthesis of ethylene from CO2 using a metal-free electrocatalyst represents a significant step towards a more sustainable future. By reducing our reliance on fossil feedstocks and minimizing CO2 emissions through innovative catalytic processes, we can work towards a more environmentally friendly and energy-efficient chemical industry. The potential impact of this research extends beyond ethylene production, as similar approaches could be applied to the conversion of CO2 into other valuable chemicals, further expanding the scope of sustainable chemical synthesis.
The development of metal-free electrocatalysts based on nitrogen-containing COFs offers a promising pathway towards the utilization of CO2 as a raw material for chemical production. By leveraging the unique properties of organic frameworks and optimizing the design of catalytic centers, researchers are paving the way for a more sustainable and efficient approach to chemical synthesis. This innovative technology has the potential to drive significant advancements in the field of green chemistry and contribute to a more sustainable future for the chemical industry.

Leave a Reply