In recent years, solar energy has gained momentum as a leading source of renewable energy in the United States. The advancements in technology have allowed for more efficient conversion of solar light into electricity, contributing to the growth of the solar industry. However, beyond electricity generation, there is a burgeoning interest in using light to drive chemical reactions. The chemical industry plays a vital role in producing essential consumer and industrial products, but it heavily relies on non-renewable energy sources for high-temperature and high-pressure processes. Approximately 40% of industrial energy use and emissions in the U.S. come from the chemicals and petrochemicals sectors, highlighting the urgent need for more sustainable methods of chemical production.
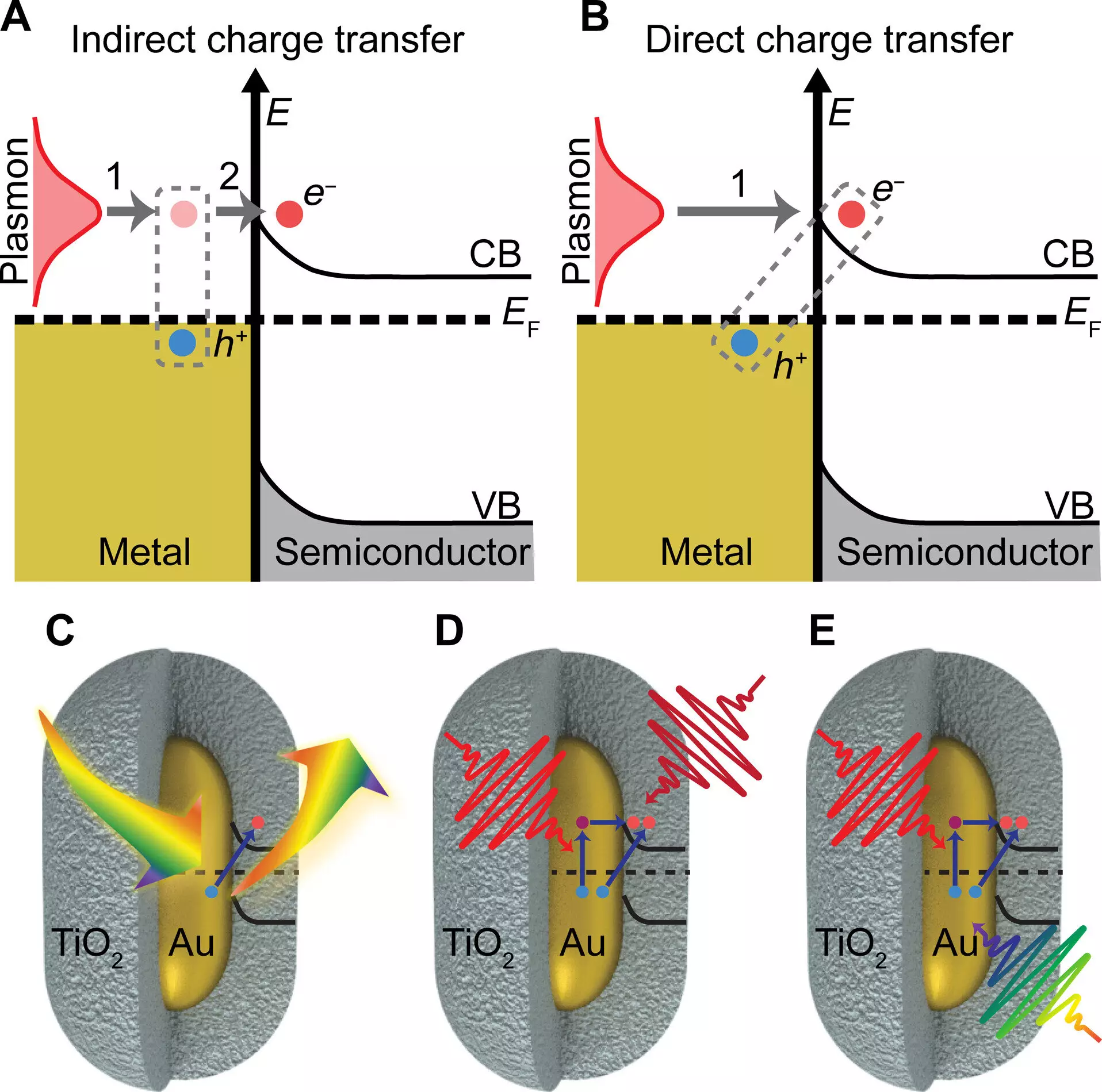
Researchers, including a team from the University of Illinois Urbana-Champaign, have been exploring the use of light, particularly sunlight, to catalyze chemical reactions. The team has uncovered a groundbreaking mechanism for charge transfer that demonstrates significantly higher efficiency compared to traditional methods. Their study, published in Science Advances, focuses on utilizing plasmonic gold nanoparticles to facilitate charge transfer to a semiconductor material, specifically titanium oxide shells. By leveraging the unique properties of gold nanoparticles, the researchers were able to quantify the amount of charge transferred using different wavelengths of light.
The integration of plasmonic nanoparticles with semiconductors holds promise for improving the efficiency of light-harvesting technologies. Gold nanoparticles, in particular, exhibit exceptional light absorption capabilities and generate collective electronic oscillations when coupled with surface plasmons. The researchers hypothesized that by exciting the plasmon with light, they could enhance the charge transfer process to the semiconductor material. Their experimental findings confirmed the theory, revealing a remarkable electron transfer efficiency of 44 ± 3% from gold nanorods to titanium oxide shells, with half of the transfer mediated by exciting the plasmon directly.
Professor Stephan Link emphasized the significance of understanding how plasmons influence charge transfer processes. The team’s research sheds light on the additional impact of plasmons in facilitating charge separation, highlighting the potential for developing more efficient devices for energy conversion. Gold nanoparticles, due to their nanoparticle size, offer a range of properties, such as tunable color and catalytic activity, which further enhance charge transfer efficiency. The researchers observed that matching the color of light absorption with the particles resulted in a substantial boost in charge transfer, indicating the importance of plasmon excitation in driving the process.
While plasmonic nanoparticles offer a promising avenue for energy conversion, challenges remain, particularly in mitigating heat generation during light absorption. Heat can compete with charge transfer processes, reducing overall efficiency. By focusing on intercepting heat generation pathways and optimizing charge transfer mechanisms, researchers aim to maximize the efficacy of light-driven chemical reactions. The study introduces a novel concept of direct plasmon-induced charge transfer, drawing from a theory known as chemical interface damping of plasmons from the 1990s. The interdisciplinary approach adopted by the researchers, combining multiple imaging and spectroscopy techniques, offers a comprehensive understanding of this phenomenon.
The research conducted by the University of Illinois Urbana-Champaign team marks a significant step towards revolutionizing chemical reactions using light. By unraveling the intricate mechanisms of plasmon-induced charge transfer, researchers are paving the way for more sustainable and energy-efficient chemical production processes. The integration of plasmonic nanoparticles with semiconductors presents exciting opportunities for enhancing light-harvesting technologies and driving advances in renewable energy solutions. As the field of plasmonics continues to evolve, future studies will explore new avenues for leveraging light to catalyze chemical reactions, with the ultimate goal of achieving sustainable and environmentally friendly industrial practices.

Leave a Reply