In a groundbreaking study published in Nature Communications, researchers from the Interface Science Department at the Fritz Haber Institute have introduced a new advancement in the fight against climate change. The study, titled “Reversible metal cluster formation on Nitrogen-doped carbon controlling electrocatalyst particle size with subnanometer accuracy,” explores a novel method for understanding the mechanisms of carbon dioxide (CO2) re-utilization to create fuels and chemicals. This research opens up the possibility for further optimization of catalytic processes powered by renewable electricity.
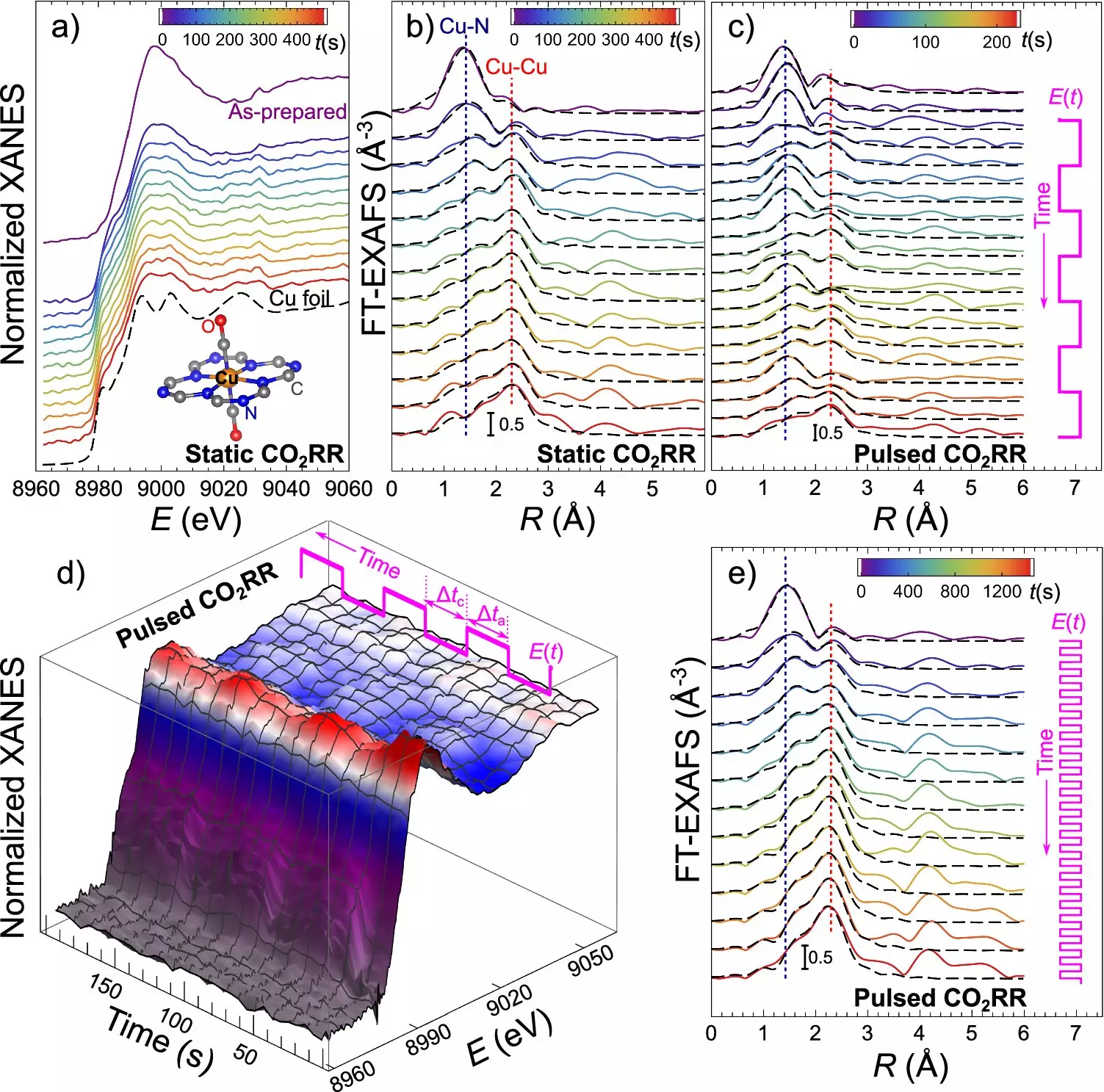
The core of this discovery lies in the unique properties of catalysts made up of ultradispersed copper and nitrogen atoms embedded in carbon. These catalysts exhibit a dynamic behavior during the electrocatalytic CO2 reduction (CO2RR) process. They can transition from single atoms of copper to small clusters and metal nanoparticles, and then revert back when the electrical potential is altered. This reversible transformation provides a crucial key to controlling the structure of the catalyst, which, in turn, dictates the outcome of the CO2RR process and influences product selectivity.
Controlling the size and structure of catalyst particles has been a major challenge in scaling up CO2RR technology for practical applications. The ability to manipulate the catalyst’s state allows for precise control over the distribution of CO2RR products. By adjusting the duration of electrical pulses, researchers are able to steer the formation of nanoparticles and determine whether the catalyst remains as single atoms, small clusters, or larger metallic copper nanoparticles. Each form of the catalyst is tailored to produce specific CO2RR products, such as hydrogen, methane, or ethylene.
To monitor and adjust the catalyst’s transformation in real-time, the research team utilized operando quick X-ray absorption spectroscopy. This cutting-edge technique enables scientists to observe the catalyst’s changes during the reaction with sub-second time resolution, ensuring the ideal conditions for the desired CO2RR products. The study not only deepens our understanding of catalyst behavior but also highlights the significant impact that controlling the catalyst’s structure can have on the CO2 reduction process.
While this research showcases potential pathways for technological applications in greenhouse gas reduction and the production of sustainable chemicals and fuels, its primary contribution lies in advancing scientific knowledge. By shedding light on the dynamic structural transformations of catalysts and their influence on the CO2RR process, this study sets the stage for future developments in the field of green chemistry. It offers a glimpse into a promising future where precise control over catalyst structure plays a key role in combating climate change and driving sustainable innovation.

Leave a Reply