In a groundbreaking development reported in the Journal of the American Chemical Society, a team of researchers has introduced a new technology aimed at overcoming the limitations of current catalyst electrodes. This innovation has paved the way for the large-scale production of green hydrogen at a relatively low cost. Led by Professor Han Gi Chae and Professor Jong-Beom Baek at UNIST, along with Professor Kafer T. Tavuz at King Abdullah University of Science and Technology (KAUST), the team has made significant strides in the field of electrocatalysis.
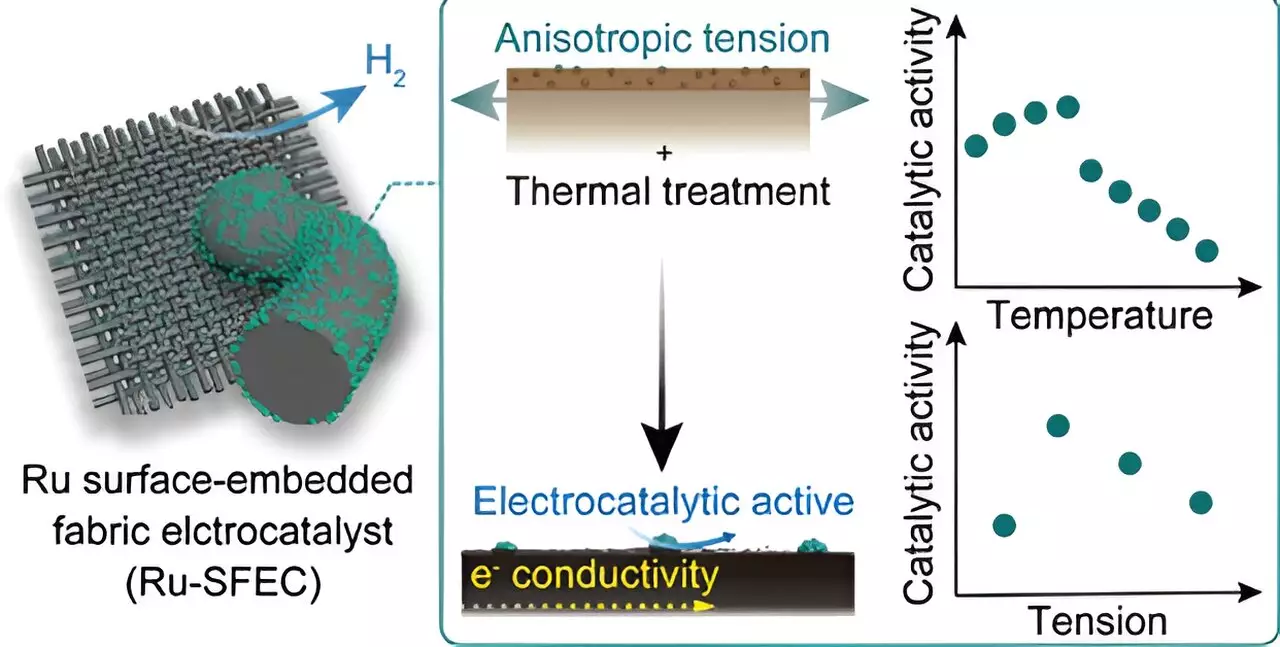
The researchers have devised a cutting-edge approach by embedding highly functional catalysts into carbon fabric electrodes. This unique design allows for stable operation across large areas, a marked improvement over traditional powder-type catalysts that are prone to detachment. By utilizing a carbon fiber catalyst, the team has achieved a lifespan for the electrode that is 100 times longer than conventional electrodes. Moreover, the use of ruthenium instead of platinum has resulted in substantially reduced manufacturing costs.
Traditionally, electrochemical electrodes were manufactured by spraying powder catalysts onto the electrode surface, leading to uneven application and issues such as clumping. The shift towards carbon fiber-based electrodes has been instrumental in addressing these challenges, thanks to the material’s high thermal and electrical conductivity properties. By integrating ruthenium into the polymer precursor fiber during the manufacturing process, the team has significantly enhanced catalyst stability, ensuring optimal performance over extended periods.
The ruthenium surface-embedded fabric electrocatalysts (Ru-SFECs) developed by the researchers have demonstrated exceptional efficiency, with a low overvoltage of 11.9 mV at a current density of 10 mA cm-2. This indicates minimal energy consumption during the hydrogen generation process. Moreover, the electrode has shown a negligible overvoltage increase of only 6.5% even after 10,000 operations, surpassing commercialized platinum powder catalysts. By utilizing ruthenium instead of platinum and incorporating it into the polymer precursor fiber early in the manufacturing process, the team has achieved a significant cost advantage for the electrode.
The innovative approach taken by the research team highlights the potential of carbon fibers as a versatile material for future electrochemical reactions. By carefully controlling catalyst metal separation and microcarbon structure, the researchers have maximized stability and activity, enabling the continuous production of catalyst fibers for direct industrial applications. This technology not only offers energy-efficient manufacturing processes but also reduces waste production, aligning with the growing demand for sustainable solutions.
This study sets a solid foundation for developing stable, binder-free, and flexible electrocatalytic electrodes that hold promise for various catalytic reactions with different metals. Future research efforts should focus on enhancing mechanical durability, electrical conductivity, and overall cost-effectiveness. With the successful validation of their findings in a commercial manufacturing process, the team’s work represents a critical advancement in the field of electrocatalysis with far-reaching implications for the future of green hydrogen production.

Leave a Reply