Anodes used for the electrolytic splitting of water have long relied on iridium-based materials. Although iridium has proven to be an effective catalyst for this reaction, its stability is a concern as iridium gradually dissolves in the electrolysis cell’s acidic environment, diminishing the catalytic effect. In an effort to enhance the stability of iridium catalysts, researchers at HZB and HI-ERN have developed a unique approach through the creation of a material library, systematically varying the concentration of iridium and titanium oxides. This innovative study has discovered that the addition of titanium oxides can significantly increase the stability of iridium, offering new possibilities for green hydrogen production and energy storage.
Titanium oxide, while not catalytically active, is known for its exceptional stability. The researchers aimed to investigate if titanium oxide could enhance the stability of iridium catalysts without compromising its catalytic effect. By producing a thin-film materials library with varying proportions of iridium and titanium oxide, they were able to evaluate the impact of different mixing ratios.
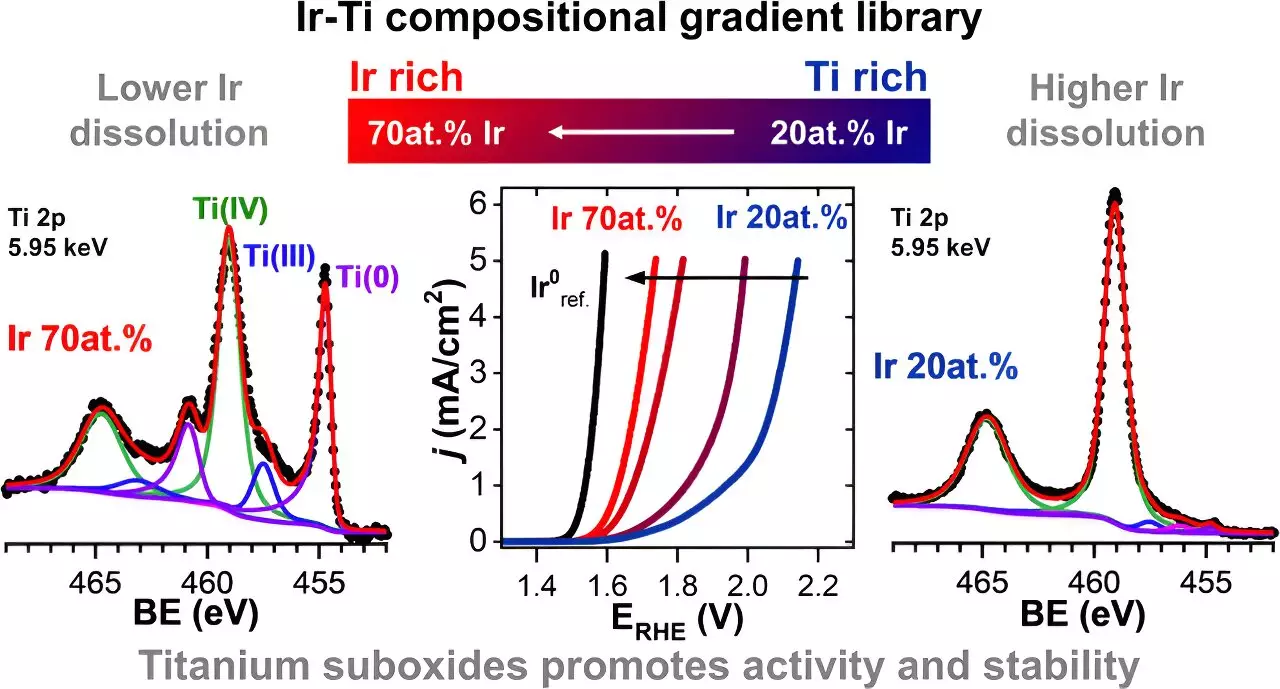
Using X-ray spectroscopic methods at BESSY II, the researchers analyzed the chemical structure of the mixed iridium-titanium oxide samples and observed notable effects. One such effect was the improved conductivity of the material due to the presence of titanium suboxides (e.g., TiO and TiOx). Additionally, some of the titanium oxides dissolved at a faster rate in the aqueous electrolyte compared to iridium, creating micropores on the material’s surface. This phenomenon facilitated the oxygen evolution reaction by allowing more iridium atoms from the lower layers to come into contact with the electrolyte. Importantly, titanium oxides (including TiO2, TiO, and TiOx) were found to significantly reduce the dissolution of iridium, leading to increased stability.
During the measurement process, it was observed that the addition of 30% titanium to the iridium electrode material resulted in an approximately 70% reduction in iridium dissolution compared to pure iridium electrodes. This finding not only highlights the significant impact of titanium oxides on stability but also suggests the possibility of an ideal mixing ratio for the highest stability and catalytic efficiency.
One key consideration in the transition from laboratory research to industrial applications is the disruption caused by altering established technologies. As such, initial adoption of these innovative findings may be challenging. However, the potential for improved stability and efficiency in water electrolysis offers promising opportunities for the industry. By incorporating titanium oxides into iridium catalysts, manufacturers can overcome the limitations of iridium dissolution and enhance the overall performance of electrolysis cells.
The development of this material library and the subsequent analysis of the iridium-titanium oxide samples have opened up new avenues for research in the field of water electrolysis. The discovery of titanium oxides’ positive effect on stability without compromising catalytic performance provides a solid foundation for further exploration and optimization of catalyst composition. Future studies could focus on finding the optimal mixing ratio of iridium and titanium oxides, as well as investigating the long-term stability and durability of these enhanced catalysts.
The quest for efficient and stable water electrolysis is essential for the widespread adoption of green hydrogen production. Through the systematic variation of iridium and titanium oxide concentrations, researchers have discovered that the inclusion of titanium oxides can significantly enhance the stability of iridium catalysts. This finding offers renewed hope for the industry as it strives to improve the performance and durability of electrolysis cells. With continued research and development, the future of water electrolysis looks promising, unleashing the full potential of “green” hydrogen as a clean and sustainable energy source.

Leave a Reply