Fuel cells have emerged as a promising carbon-free energy source as the world works towards transitioning away from fossil fuels. These cells consist of an anode, a cathode, and an electrolyte, all working together to convert the chemical energy of fuel directly into electricity. The process involves oxidation of fuel at the anode and reduction of oxygen at the cathode, resulting in the production of electricity and water as the only byproduct. However, the efficiency of fuel cells can be hindered by the presence of water, which can lead to energy losses and increased costs due to the high platinum loading required to maintain efficient operation.
A recent study conducted by Professor Nagahiro Hoshi and his team from the Graduate School of Engineering at Chiba University, Japan, has made a groundbreaking discovery regarding the use of caffeine to improve the efficiency of fuel cells. The researchers found that adding caffeine to certain platinum electrodes can increase the activity of the oxygen reduction reaction (ORR), leading to a potential reduction in platinum requirements and making fuel cells more affordable and efficient.
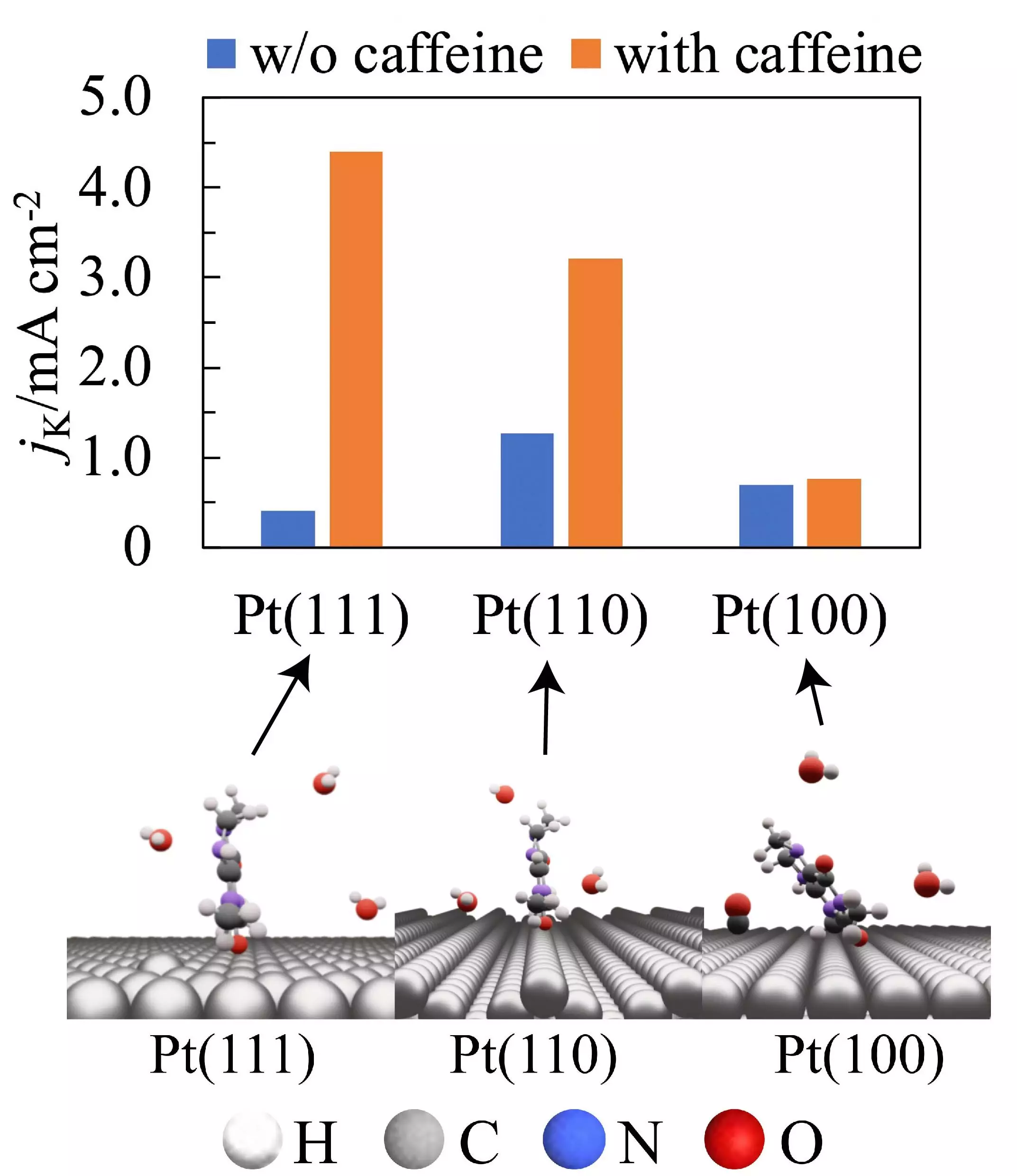
The study revealed that caffeine, a compound found in coffee, can enhance the activity of the ORR by 11-fold on a well-defined platinum electrode with a hexagonal atomic structure. By measuring the current flow through platinum electrodes immersed in an electrolyte containing caffeine, the researchers observed a significant improvement in the ORR activity with an increase in caffeine concentration. The presence of caffeine on the electrode’s surface effectively prevented hydrogen adsorption and the formation of platinum oxide, ultimately increasing the efficiency of the fuel cell.
The researchers discovered that the impact of caffeine on the ORR activity varied depending on the orientation of platinum atoms on the electrode’s surface. Caffeine was found to adsorb onto Pt(111) and Pt(110) surfaces with its molecular plane perpendicular to the surface, leading to a decrease in platinum hydroxide coverage and lower steric hindrance of the adsorbed caffeine. This resulted in a significant increase in ORR activity on Pt(111) and Pt(110) electrodes. However, on Pt(100) surfaces, steric hindrances caused caffeine to be attached with its molecular plane tilted relative to the surface, counteracting the effect on ORR activity.
Unlike traditional batteries with limited lifespans, fuel cells can generate power as long as fuel is supplied, making them a viable energy source for various applications, including vehicles, buildings, and space missions. The discovery of caffeine’s impact on fuel cell efficiency has the potential to revolutionize the design and widespread use of fuel cells, ultimately contributing to a more sustainable and cost-effective energy future.

Leave a Reply