The recent advancements in research have paved the way for developing novel substances with modified structural motifs commonly found in pharmaceuticals. Sustainable chemical transformations have gained significant importance globally across various industries. Electrochemistry has emerged as a key player in driving the development of sustainable synthetic routes, thereby reducing the generation of waste associated with traditional chemical transformations.
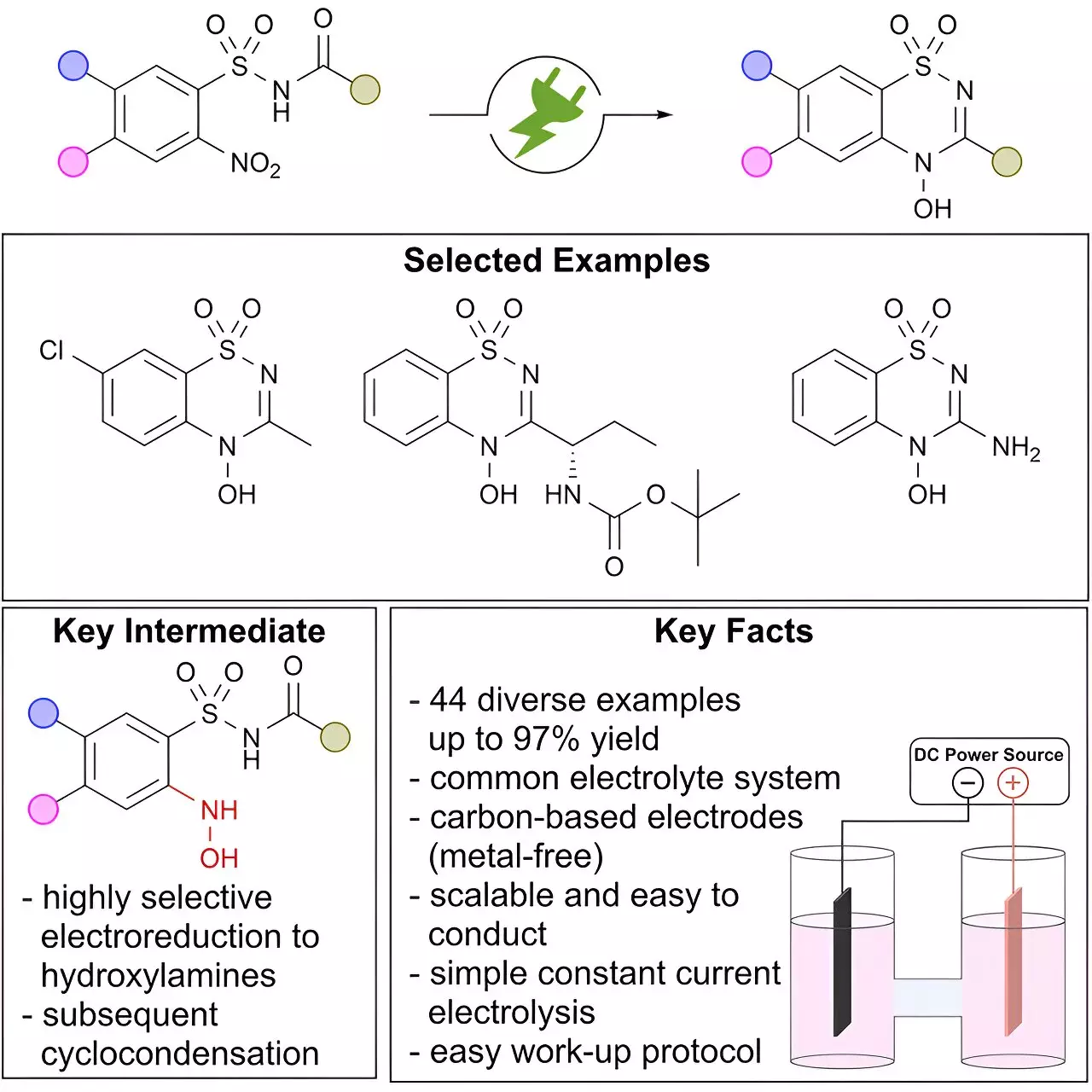
Electro-organic synthesis has been identified as a crucial method in avoiding the generation of large amounts of waste in chemical processes. A team of scientists from the Max Planck Institute of Chemical Energy Conversion (MPI CEC) conducted a study to showcase how electrochemistry is revolutionizing the modern organic chemist’s toolkit. This study demonstrated that the electrochemical conversion of nitro groups, which typically requires significant quantities of reductants or rare metals, can provide direct access to a previously unexplored class of N-hydroxy heterocycles, offering a new structural motif for drug discovery.
Nitrogen-containing heterocycles, specifically benzo[e]-1,2,4-thiadiazine-1,1-dioxides, are widely utilized in blockbuster pharmaceuticals like Diazoxide. However, there is limited knowledge about heterocycles containing an exocyclic N-hydroxy modification. The unique stability and characteristics of the N-O bond in the N-hydroxy motif make it particularly intriguing for pharmaceutical research. Additionally, N-oxy heterocycles are frequently identified as major drug metabolites, further highlighting the importance of exploring this class of compounds.
Siegfried R. Waldvogel and his team at MPI CEC and Johannes Gutenberg-Universität Mainz have demonstrated the effectiveness of electro-organic synthesis in selectively producing N-hydroxy derivatives through the cathodic reduction of nitro arenes. This innovative approach has facilitated the scalable and highly selective synthesis of benzo[e]-1,2,4-thiadiazine-1,1-dioxides, a critical structural motif found in various APIs enhanced with a unique N-hydroxy moiety. While this study has expanded the compound library of benzo[e]-1,2,4-thiadiazine-1,1-dioxides, the biological properties of these compounds remain largely unknown.
Further investigation into the biological properties of the synthesized compounds could unlock potential implications for improving drug efficiency, understanding metabolism, and exploring novel pharmaceutical applications. The findings from this study underscore the transformative potential of electrochemistry in sustainable chemical transformations and its significant contributions to modern drug discovery and development.

Leave a Reply