The field of photocatalytic hydrogen evolution from water is a critical component in achieving sustainable hydrogen production. A recent study published in the Journal of the American Chemical Society delves into the microscopic structure of interfacial water molecules and their impact on photocatalytic reactivity. The study, titled “Positive and negative impacts of interfacial hydrogen bonds on photocatalytic hydrogen evolution,” sheds light on the crucial roles of interfacial hydrogen bond structure and dynamics in promoting H2 evolution.
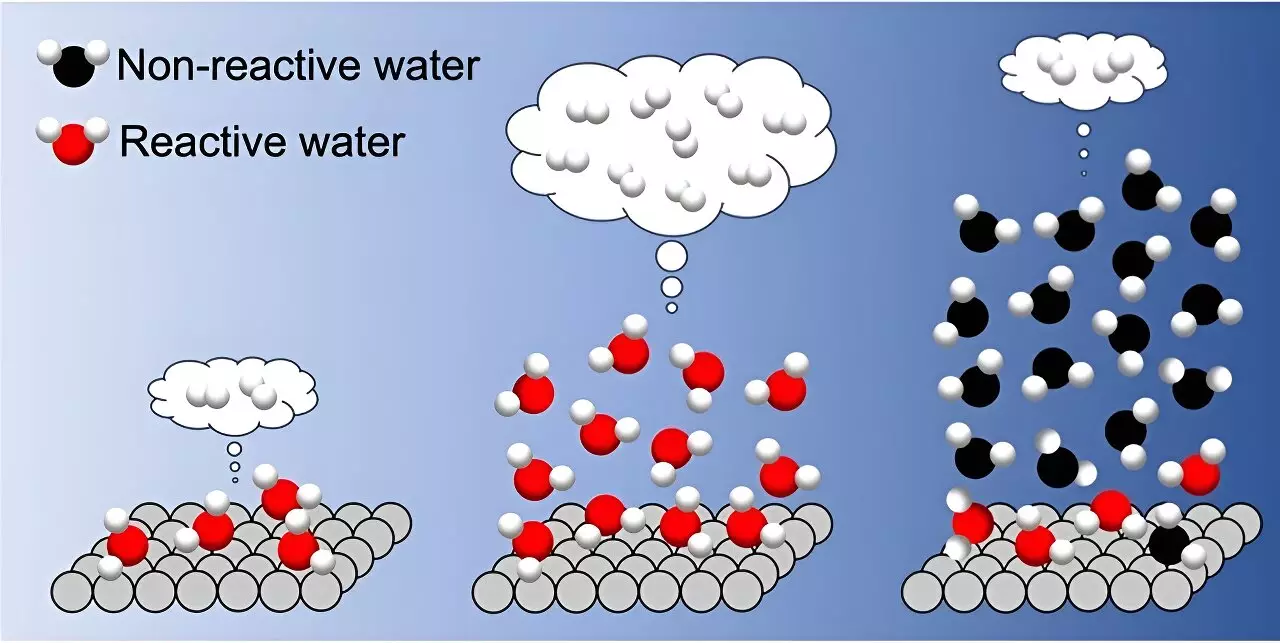
Researchers, led by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, comprehensively investigated the impact of interfacial hydrogen bond networks using various TiO2 photocatalysts. Through precise control of the thickness of adsorbed water, they were able to demonstrate a direct correlation between H2 formation rate and the microscopic structure of hydrogen bond networks. The study revealed that while reactive water molecules in the first adsorbed layer contribute to hydrogen evolution, the presence of liquid-like water adsorbed in more than three layers hinders interfacial proton-coupled hole transfer and decreases the H2 formation rate.
The study suggests that depositing three water layers in a water vapor environment is optimal for photocatalytic hydrogen evolution. This approach represents a potential paradigm shift in the field of photocatalysis, as it demonstrates the effectiveness of water vapor environments compared to traditional liquid-phase reaction systems. By providing molecular-level insights into the design of interfacial water conditions, researchers aim to enhance photocatalytic performance and achieve breakthroughs in sustainable hydrogen production.
Photocatalysis has been a subject of extensive research for over half a century, primarily in aqueous solution environments. The findings of this study open up new avenues for the design and engineering of interfacial water in photocatalytic systems for next-generation renewable energy production. By understanding the physicochemical properties of interfacial water molecules and their hydrogen bond networks, researchers can work towards developing more innovative photocatalytic systems that contribute to the advancement of sustainable energy solutions.
The study highlights the importance of interfacial hydrogen bond structure and dynamics in optimizing photocatalytic efficiency for hydrogen evolution from water. By uncovering the roles of interfacial water molecules in promoting H2 evolution, researchers have provided valuable insights that can guide the design of more efficient photocatalytic systems. This research represents a significant step towards achieving sustainable hydrogen production through the utilization of light energy at room temperature.

Leave a Reply