Calcite, a crystalline form of calcium carbonate, is a mineral that is commonly found in limestone and marble. It is recognized by its rhombohedral appearance, resembling a distorted cube under a microscope. While calcite is widely abundant on Earth, its properties and internal structure have significant implications in various fields of research.
Calcite plays a crucial role in the transformation of carbon dioxide (CO2) into solid carbonate, which is essential for long-term carbon storage to combat climate change. Recent research has shown that the presence of defects in calcite can be controlled through synthesis approaches, affecting its reactivity and properties. These defects have the potential to impact the absorption of harmful substances like heavy metals and influence the mechanical strength of materials.
A recent study conducted by researchers at the U.S. Department of Energy’s Argonne National Laboratory revealed the internal nanocrystallinity of calcite due to nonclassical crystallization. The researchers discovered that the method of synthesis dramatically alters the internal structure of individual calcite particles, which in turn affects their reactivity.
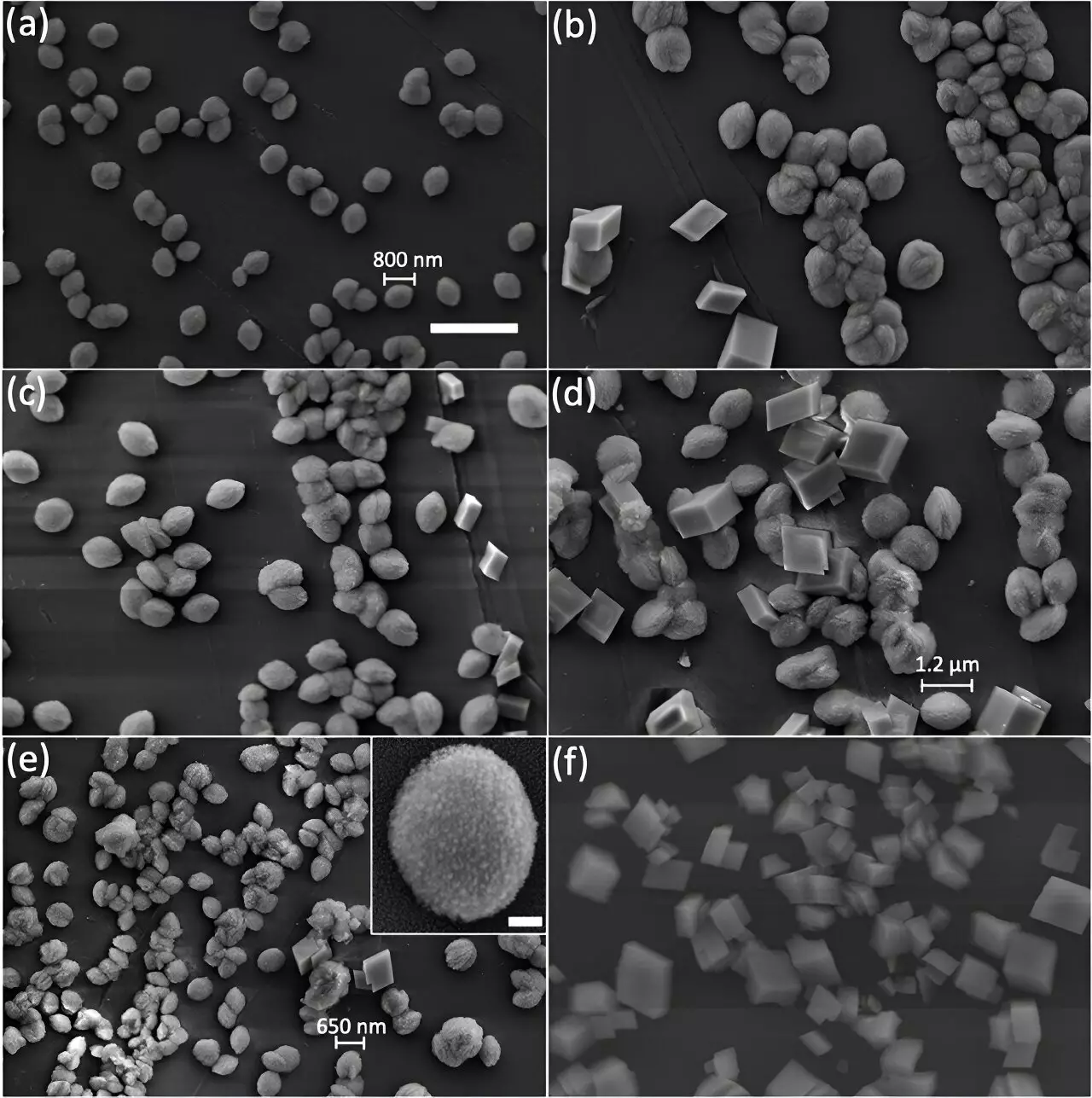
To investigate the internal structure of calcite particles, researchers used advanced imaging techniques such as scanning electron microscopy (SEM), powder X-ray diffraction, and Bragg Coherent Diffraction Imaging (BCDI). The BCDI experiment provided a high-resolution view of calcite’s internal crystallinity by capturing localized features within individual mineral particles.
The study compared calcite particles grown by two different synthesis approaches – slow growth and rapid growth. While SEM images of slowly grown crystals displayed expected patterns, those grown rapidly revealed a complex internal structure with nanosized crystalline fragments or defects. These defects altered the reactivity of calcite and demonstrated the unique properties of fragmented particles.
The presence of nanoscopic defects in calcite indicates granular substructures similar to vaterite, another form of calcium carbonate. Understanding how internal defects affect calcite reactivity is essential for distinguishing between perfect particles and fragmented ones. These defects can significantly impact the chemical reactions and functionality of calcite, particularly in terms of absorbing toxic substances like heavy metals.
By identifying and studying internal fragmentation in the crystalline structure of minerals, researchers can explore the potential for designing materials with optimized strength and toughness. The findings from this study may extend to other fields such as catalysis, where the presence of fragments inside particles could enhance catalytic activities by increasing reactive surface areas. Imaging techniques like BCDI provide a direct method for examining calcite features and establishing structure-property relationships.
The internal structure of calcite and its sensitivity to synthesis approaches highlight the importance of understanding how defects and fragmentation can influence its reactivity and properties. By further exploring these mechanisms, researchers can advance the development of materials with tailored functionalities and applications across various scientific disciplines.

Leave a Reply