Alcoholic beverages have been consumed for centuries, and each type of drink has its own ideal serving temperature. Researchers have found that the taste of alcoholic beverages can be influenced by temperature, specifically in terms of how “ethanol-like” they taste. This study suggests that the formation of different clusters of water and ethanol molecules at varying temperatures can affect the perceived taste of alcohol.
Lead author Lei Jiang and his team set out to investigate the relationship between temperature and alcohol taste perception. Their research was inspired by a simple question posed by Jiang’s colleague, Xiaotao Yang, during a casual beer-drinking session. This initial curiosity led them to explore how water and ethanol molecules interact at the molecular level and how this interaction translates to the taste experience.
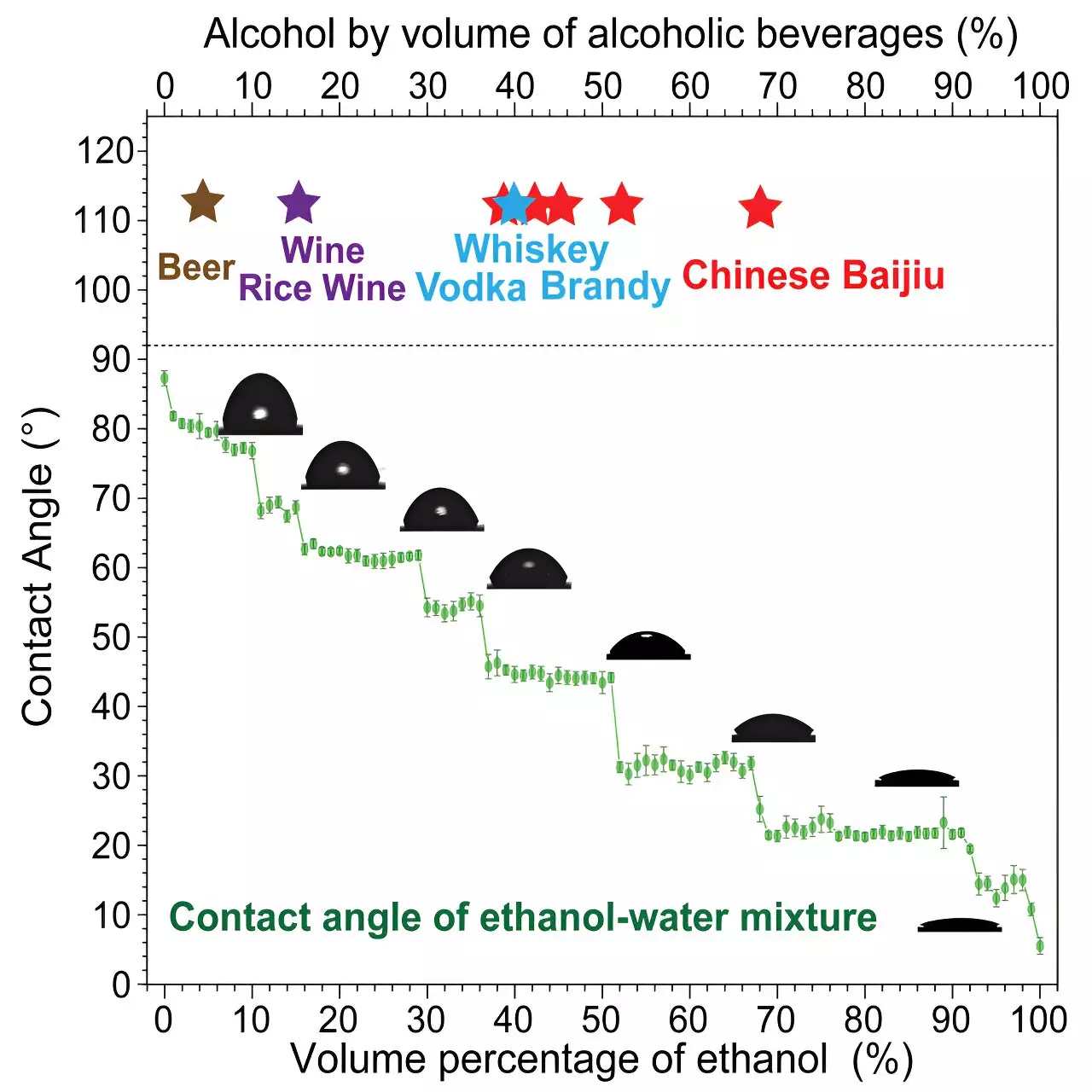
The team began by measuring the contact angle of various alcohol-water solutions to understand how different concentrations of alcohol affect the behavior of liquid molecules. They found that the contact angle did not increase linearly with increasing alcohol concentration, but instead, it showed irregular plateaus as the concentration rose. This unexpected observation hinted at the formation of distinct clusters of ethanol and water molecules in the solution.
Further experiments revealed that at low ethanol concentrations, ethanol tended to form pyramid-shaped structures around water molecules. However, as the ethanol concentration increased, the molecules aligned in a chain-like fashion. Interestingly, these cluster structures changed when the solutions were cooled or heated, leading to different taste perceptions of alcohol.
The researchers believe that their findings could have practical applications in the alcoholic beverage industry. By understanding how different cluster structures influence taste perception, beverage manufacturers may be able to manipulate temperature to achieve specific taste profiles with the lowest ethanol concentration possible. This could be particularly useful for products like baijiu, which have distinct alcohol concentrations.
For example, the study noted that baijiu at 51% alcohol content tasted noticeably different from baijiu at 52%, despite only a 1% difference in alcohol concentration. This difference in taste perception was attributed to the arrangement of ethanol molecules in the solution. Similarly, beer tasted more “ethanol-like” when chilled, as colder temperatures favored the formation of chain-like structures in lower ethanol concentration solutions.
Future Research and Industry Applications
The findings of this study open up new avenues for exploring the impact of temperature on alcohol taste perception. By delving deeper into how water and ethanol molecules interact at different temperatures, researchers may uncover novel ways to enhance or modify the taste of alcoholic beverages. Additionally, the beverage industry could leverage this knowledge to develop products with specific taste profiles that cater to diverse consumer preferences.
The study sheds light on the intricate relationship between temperature and alcohol taste perception. By examining the molecular behavior of ethanol and water molecules in solution, researchers have uncovered valuable insights into how temperature influences the taste of alcoholic beverages. This research has the potential to revolutionize both the scientific understanding of taste perception and the practical applications within the beverage industry.

Leave a Reply