The atomic bombing of Hiroshima, Japan, by the United States in August 1945 left a devastating impact not only on the city and its residents at the time but also had long-standing consequences that continue to be felt to this day. One of the significant findings from continued research in Hiroshima Bay is the discovery of a new type of debris known as Hiroshima glasses. These glasses formed from the vaporized materials of the bomb and the surrounding landscape and infrastructure.
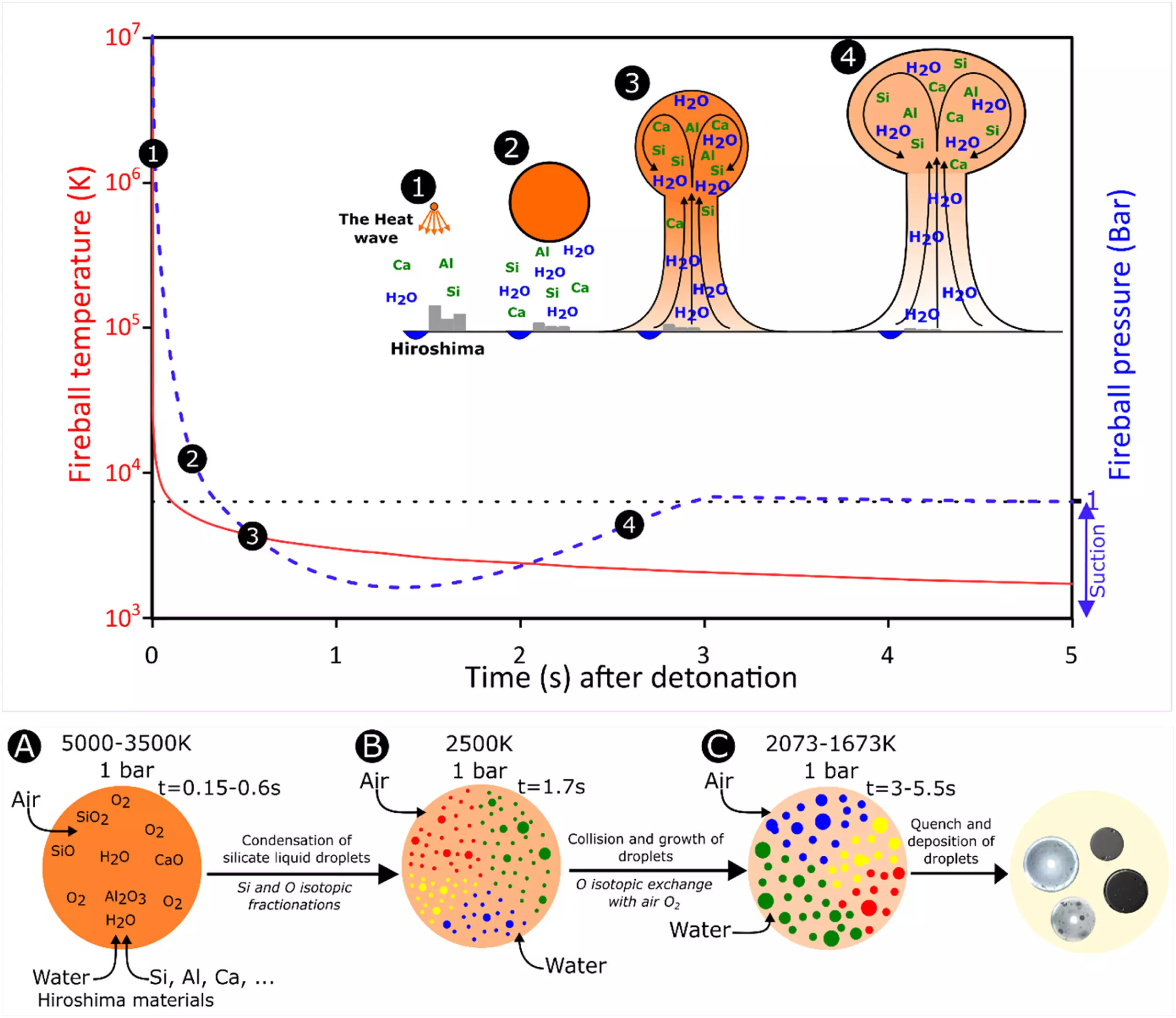
Recent research published in Earth and Planetary Science Letters has focused on analyzing the chemical and isotopic compositions of these Hiroshima glasses to understand their formation process during the nuclear event. Nathan Asset and colleagues from Université Paris Citè in France discovered that rapid condensation occurring within the nuclear fireball, which lasted between 1.5 to 5.5 seconds at temperatures ranging from 3,200 to 1,000 Kelvin, was the primary process responsible for the formation of these glasses. This process bears resemblance to the formation of the first solids in the solar system, known as calcium-aluminum-rich inclusions (CAIs) found in primitive meteorites.
The research team identified four types of glasses within the 94 specimens of fallout debris collected from Hiroshima. These included melilitic glasses, characterized by low silica content, high calcium oxide, and magnesium oxide; anorthositic glasses with high aluminum oxide and iron content; soda-lime glasses rich in silica and sodium oxide; and nearly pure silica glasses. While the origin of the silica glass could not be distinguished from sand grains on the beach, the soda-lime glasses displayed similarities to compositions of industrial origin.
Read More: The Mysteries of Black Holes Unveiled: A New Perspective
By reconstructing the formation of these glasses, the researchers determined that the plasma fireball exploded 580 meters above the city with a radius of 260 meters, reaching peak temperatures of 107 Kelvin and a pressure of 10^6 atmospheres. A thermal wave touched the ground with temperatures as high as 6,287°C, causing various city materials such as concrete, iron, aluminum alloys, industrial glass, and soil to vaporize within a fraction of seconds and mix with sand, river water, and the atmosphere to produce the different glasses.
The isotopic composition of silica within the Hiroshima glasses showed values ranging from -23.0 ± 1.8 ‰ to -1.5 ± 1.1 ‰, while that of oxygen displayed mass-independent fractionation values of -3.1 ± 0.6 ‰, all falling within the composition ranges of CAIs. Through the results of fractionation, the research team concluded that melilitic glasses were the first to form, followed by anorthositic, soda-lime, and finally nearly pure silica glasses.
Despite the differences in environment composition and formation time between Hiroshima glasses and CAIs, understanding the processes that occurred during the gas-solid transition provides valuable insights into the origins of our solar system and the development of various celestial bodies. By studying the formation of Hiroshima glasses, researchers can unravel the mysteries of the early solar system and gain a deeper understanding of how our world came to be.

Leave a Reply