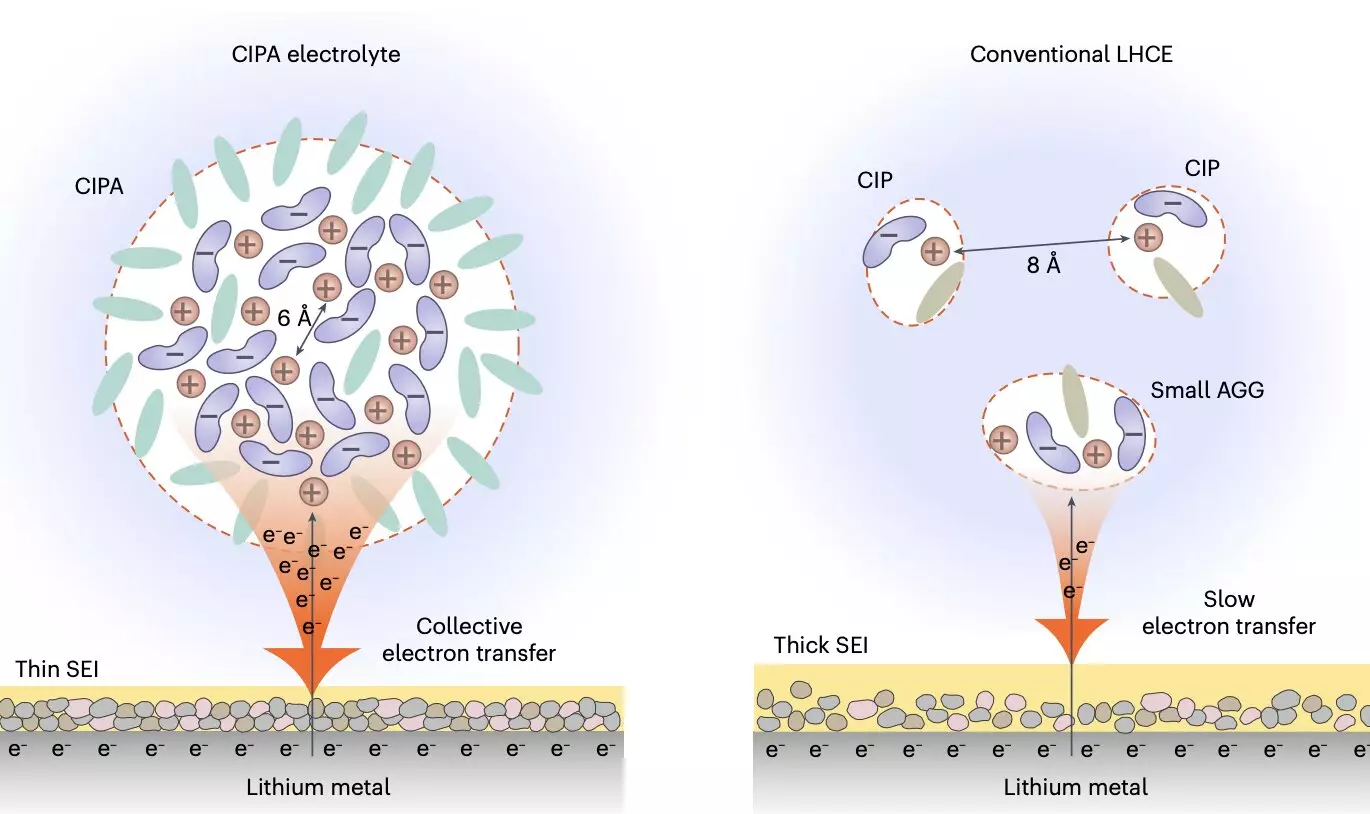
One of the most promising advancements in battery technology is the development of lithium-metal batteries, which offer significantly higher energy densities compared to the current lithium-ion batteries dominating the market. However, these lithium-metal cells face challenges, particularly in terms of lifespan and cycle life. Researchers from the University of Science and Technology of China have recently introduced a novel electrolyte design that shows promise in addressing these limitations. This innovative electrolyte features a nanometer-scale solvation structure, comprising densely packed ion-pair aggregates (CIPA) that could lead to the development of high-performing lithium-metal pouch cells with extended lifespans.
While lithium-metal batteries hold the potential for ultra-high energy density, the current cycle life of these batteries is around 50 cycles, significantly lower than commercial lithium-ion batteries that can last approximately 1,000 cycles. The primary factors contributing to the limited lifespan of lithium-metal batteries include the formation of lithium dendrites, the reactivity of lithium-metal, and the degradation of the electrolyte. These challenges have hindered the practical application of lithium-metal batteries despite their promising energy storage capabilities.
Five years ago, researchers began exploring innovative electrolyte designs to stabilize the interfaces between the electrolyte and the electrodes in lithium-metal batteries. By enhancing the anode-electrolyte and cathode-electrolyte interfaces, researchers aimed to suppress electrolyte degradation and improve the overall performance of lithium-metal cells. One crucial aspect of these advances is the solvation structure of the electrolyte, which plays a critical role in determining the behavior and stability of the battery system.
Through collaborative efforts with other research teams, Prof. Shuhong Jiao and her colleagues have made significant progress in designing a new class of electrolytes that can potentially extend the lifespan of lithium-metal batteries. These electrolytes are composed of affordable molecules and feature a unique solvation structure that focuses on ion pairs and the formation of compact ion-pair aggregates (CIPA). By tuning the mesoscopic solvation structure, researchers have achieved a breakthrough in electrolyte design for lithium-metal batteries.
The newly designed electrolyte exhibits collective reduction behavior on the lithium-metal anode, leading to the formation of stable solid-electrolyte interphase (SEI) layers that suppress electrolyte decomposition. This reduction process results in homogeneous lithium deposition, reduced dendrite formation, and improved battery performance over multiple cycles. Additionally, the electrolyte demonstrates good oxidative stability and prevents the dissolution of transition metal elements from the cathode, further enhancing the stability of the battery system.
As researchers continue to refine and optimize the electrolyte design for lithium-metal batteries, there is a growing potential to extend the cycle life of these advanced energy storage devices. The newly developed electrolyte has already shown promising results in initial tests, with a 500 Wh/kg lithium-metal pouch cell retaining 91% of its energy after 130 cycles. The research team is now focused on further enhancing the cycle life of these cells to over 1,000 cycles, paving the way for the practical implementation of high-energy-density lithium-metal batteries in various applications, including electric vehicles and portable electronics.
The innovative electrolyte design presented in this study represents a significant step forward in the field of lithium-metal battery research, offering new insights into enhancing battery performance and lifespan. By addressing critical challenges such as electrolyte degradation and electrode instability, researchers are unlocking the full potential of lithium-metal batteries as a viable next-generation energy storage solution.

Leave a Reply