Clathrate hydrates are unique water structures that contain foreign guest molecules within a host water-molecule shell. These structures form lattices of water molecules that assemble around guest substances, creating hydrogen-bonded frameworks. These frameworks, known as Frank-Kasper (FK) phases, have a geometric arrangement as close-packed tetrahedra, making them challenging to synthesize due to the weak bonds between water and guest molecules.
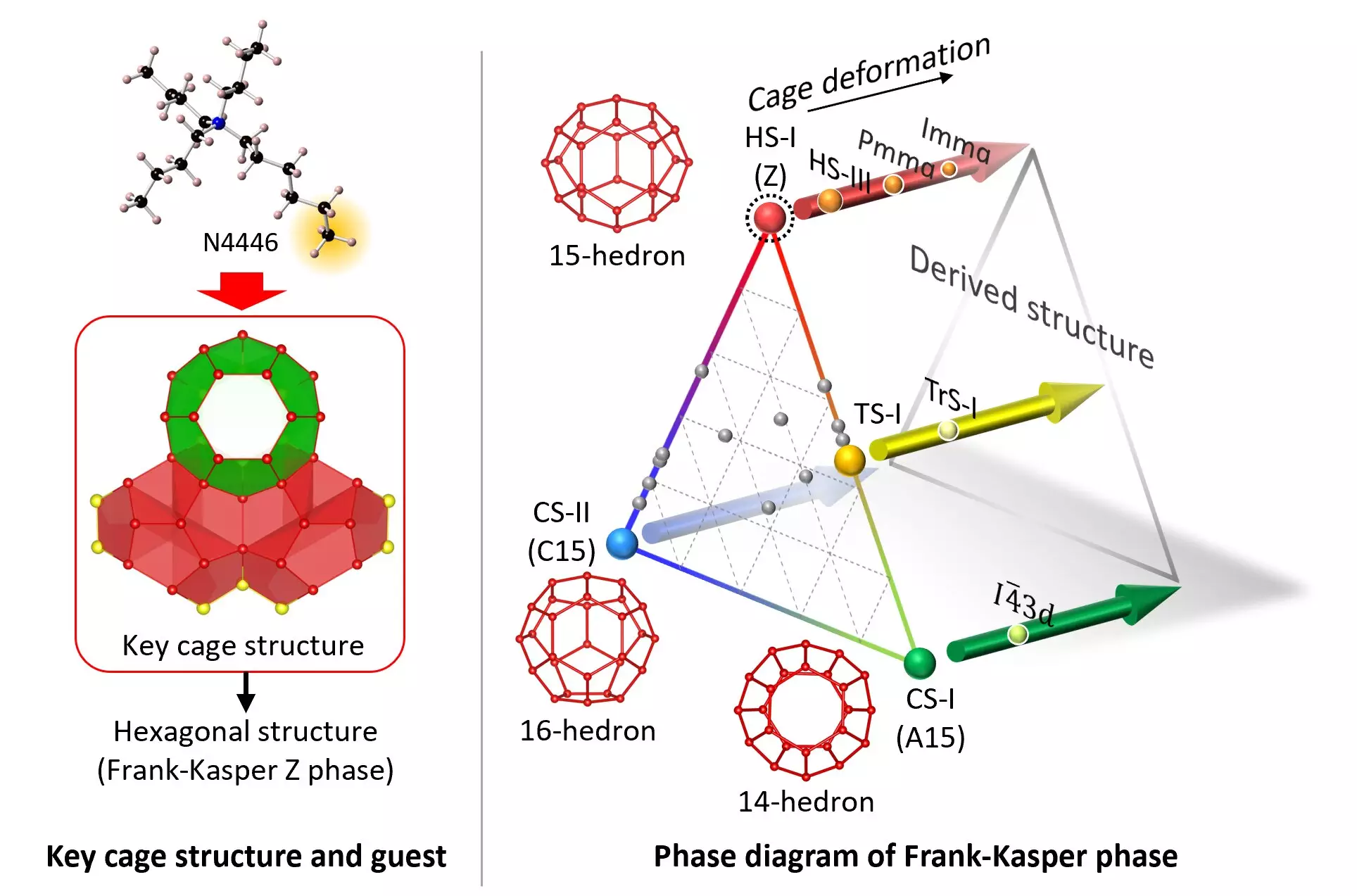
In a recent study published in Science Advances, a team of chemical engineers and crystallographers from Yokohama National University and the National Institute of Advanced Industrial and Science Technology (AIST) in Japan successfully generated a stable form of the HS-I clathrate hydrate. This phase structure, which had been previously reported as metastable, features a hexagonal crystal lattice.
The Role of Guest Molecules
The researchers were able to stabilize the HS-I clathrate hydrate by fine-tuning the guest molecule, tri-n-butyl, n-hexylammonium chloride (N4446Cl). The specific n-hexyl chain within the guest molecule played a crucial role in stabilizing the unique pentakaidecahedron water-molecule cage required for the HS-I structure. By carefully adjusting the guest molecule, the team was able to produce stable forms of the clathrate hydrate under various gas pressures.
The discovery of stable HS-I clathrate hydrate has significant implications for material science research. By engineering clathrate hydrate with mixed FK phases, researchers may be able to develop materials with properties suitable for a wide range of applications. This includes advancements in storage and transportation technologies for natural gas and synthetic fuels, carbon dioxide separation and recovery technologies, and the creation of novel materials.
One notable aspect of the research is the ability to synthesize HS-I clathrate hydrate under conditions similar to ambient temperature and pressure. This shift away from extreme conditions, such as ultrahigh pressure or ultracold temperatures, showcases the potential for applying physicochemical properties of water lattices to ecological research and simplifying material development processes. The synthesis of stable clathrate hydrate structures offers a promising avenue for further exploration in various scientific fields.
Moving forward, the team aims to explore additional FK phase structures for other compounds by refining the structures of guest molecules. The potential applications of the HS-I structure in storage, transportation, and CO2 capture technologies highlight the versatility and importance of clathrate hydrates in material science research. Continued research and development in this area hold promise for the creation of innovative materials with diverse functionalities.
The synthesis of stable forms of clathrate hydrates represents a significant advancement in material science research. By understanding the complex structures and properties of these unique water lattices, researchers can unlock new possibilities for material development and ecological research. The importance of clathrate hydrates in future applications underscores the need for continued exploration and innovation in this field.

Leave a Reply