Cholera infections, often caused by Vibrio cholerae bacteria, can have severe consequences and even be life-threatening due to the cholera toxin that is produced by these bacteria. The primary mechanism involves the toxin binding to specific “sugar lipids,” known as GM1 gangliosides, on the surface of intestinal cells. This bond is considered one of the strongest interactions between a protein and sugar molecules, allowing the toxin to enter intestinal cells rapidly and leading to significant fluid loss.
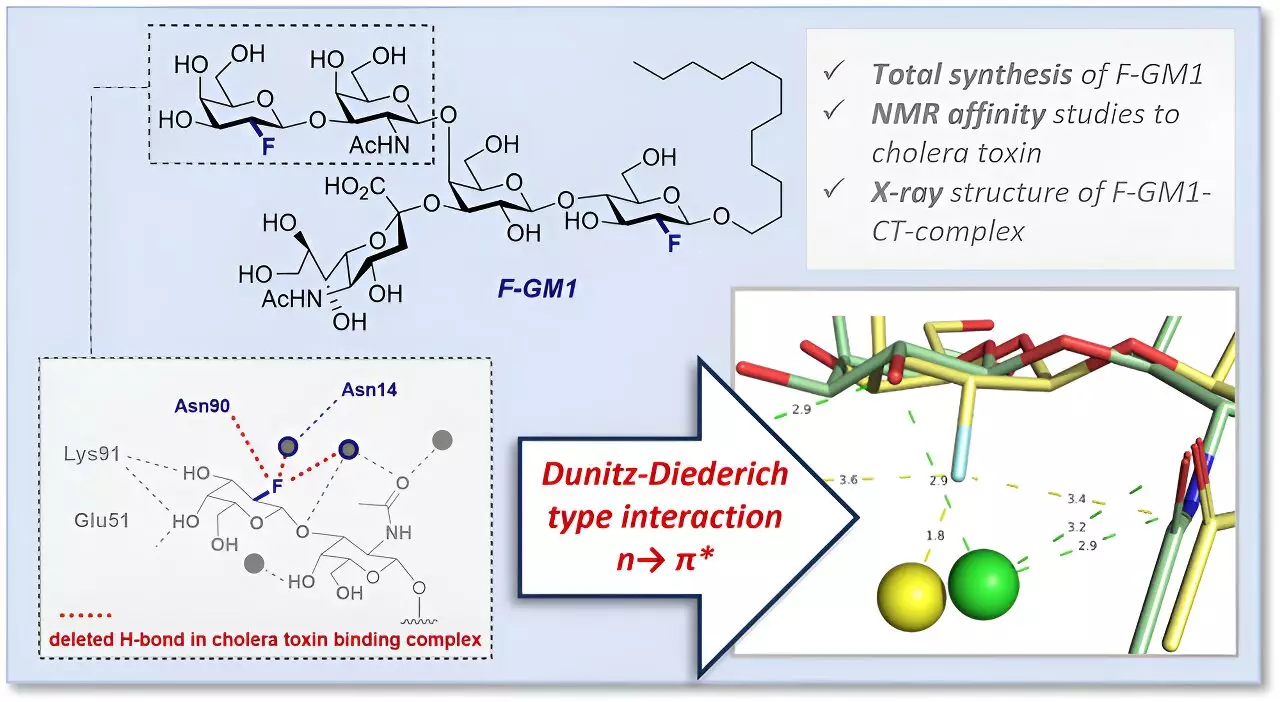
In a collaborative effort involving researchers from the University of Münster, ETH Zürich, and Leibniz Universität Hannover, a key component of the GM1 cholera toxin complex was analyzed for the first time using a fluorinated GM1 analog. This innovative approach sheds light on the molecular mechanisms behind the strong interaction between GM1 gangliosides and the cholera toxin, providing valuable insights into the disease and potentially opening doors for the development of new drugs.
Carbohydrates are vital biomolecules that play a crucial role in various biological and medical processes. They are involved in functions such as determining blood groups, regulating the immune system, and providing energy to cells. Despite their significance, the interactions between carbohydrates and target proteins are often too weak to be therapeutically effective. However, the notable exception lies in the powerful bond between GM1 gangliosides and cholera toxin, which has been extensively studied over the years.
To gain a more precise understanding of the molecular interactions between GM1 gangliosides and cholera toxin, researchers developed a fluorinated GM1 analog through a complex chemical synthesis. The introduction of fluorine in place of a hydroxyl group offered several advantages, including enhanced molecule stability against enzymatic degradation, control over spatial arrangement during glycosylation, and the ability to investigate interactions with the toxin in solution using NMR spectroscopy.
By employing protein crystallography, the research team delved deeper into the interactions and spatial arrangement of atoms within the binding pocket of the fluorinated GM1 analog. This detailed analysis revealed an additional interaction within the molecular complex facilitated by fluorine, along with a modified arrangement of functional groups in the binding pocket. These insights into the structure and interactions provide valuable information for understanding the slightly lower affinity of the analog for cholera toxin compared to natural GM1.
The study’s findings underscore the potential of using fluorinated gangliosides in biomedical research. This approach could be instrumental in investigating molecular signaling pathways involving sugar chains, identifying new therapeutic agents, or even contributing to the development of vaccines. The unique properties of fluorine offer exciting possibilities for further exploration in the realm of carbohydrate-protein interactions.

Leave a Reply