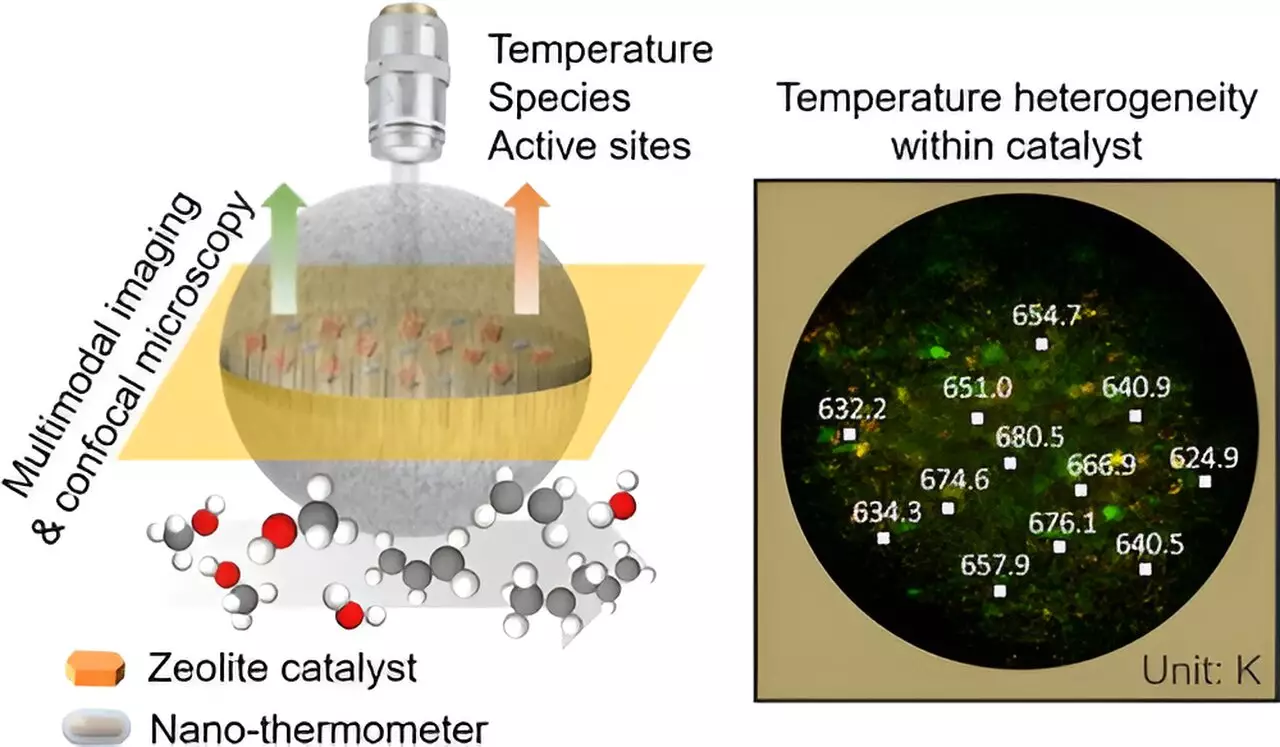
Temperature plays a crucial role in chemical reactions, affecting both thermodynamics and kinetics. In a recent study by researchers at the Dalian Institute of Chemical Physics, a groundbreaking technique was developed to measure the temperature distribution inside a single industrial zeolite-catalyst particle. This three-dimensional spatiotemporal-resolved technique allows for precise measurement of temperature near active sites, providing insights into reaction mechanisms and microscopic reaction kinetics.
Traditional methods such as thermocouples and infrared thermal imaging have limitations in measuring temperature inside catalyst particles, as they can only provide surface temperature readings with millimeter-scale spatial resolution. This poses a challenge in understanding the internal temperature dynamics of catalyst particles during reactions, especially in industrial processes where catalyst particle sizes can range from tens to hundreds of microns.
To address this challenge, the researchers developed an innovative imaging technique with a spatial resolution of 800 nm, allowing for dynamic measurement of temperature distribution inside zeolite catalyst particles during methanol-to-olefins reactions. By implanting up-conversion nano-thermometers with high-temperature resistance into catalyst particles using a microfluidic chip, the researchers were able to achieve unprecedented spatial and temporal resolution in temperature measurement.
Through multimodal imaging techniques such as confocal fluorescence and confocal infrared microscopy, the researchers investigated the effects of zeolite content and particle size on temperature distribution inside catalyst particles. The study revealed the impact of heterogeneous temperature distribution on the utilization of active sites and the evolution of reaction intermediates during methanol-to-olefins reactions. This valuable information can pave the way for the rational design and optimization of industrial catalysts and catalysis, leading to improved efficiency and yield in chemical processes.
The development of this advanced temperature measurement technique represents a significant step forward in the field of catalysis research. By enabling researchers to study temperature dynamics at a microscopic level inside catalyst particles, this new method offers valuable insights that can inform the design and optimization of industrial catalysts. With further research and application, this technique has the potential to revolutionize the way we approach chemical reactions and catalytic processes in the future.

Leave a Reply