The behavior of water molecules at an interface with air has always been a topic of interest and significance in the scientific community. Water is known for its anomalous properties, such as having higher freezing and boiling points than expected, and being less dense as a solid compared to its liquid form. These properties are attributed to the presence of weak hydrogen bonds between neighboring water molecules. These hydrogen bonds are a result of oxygen attracting electrons more than hydrogen, leading to a slightly negative charge on oxygen and a slightly positive charge on hydrogen. However, surface water molecules experience these hydrogen bonds differently than those in the bulk of liquid water.
Despite the importance of understanding the behavior of water molecules at interfaces, studying these molecules is no easy task. With the surface molecules being a tiny fraction of the total water molecules, isolating their signals and studying their behavior presents a significant challenge. Scientists have struggled to uncover how surface molecules relax after being stretched due to the complexities involved in detecting their signals. The lack of knowledge about water molecules at interfaces compared to those in the bulk liquid has been a longstanding issue in the field of molecular spectroscopy.
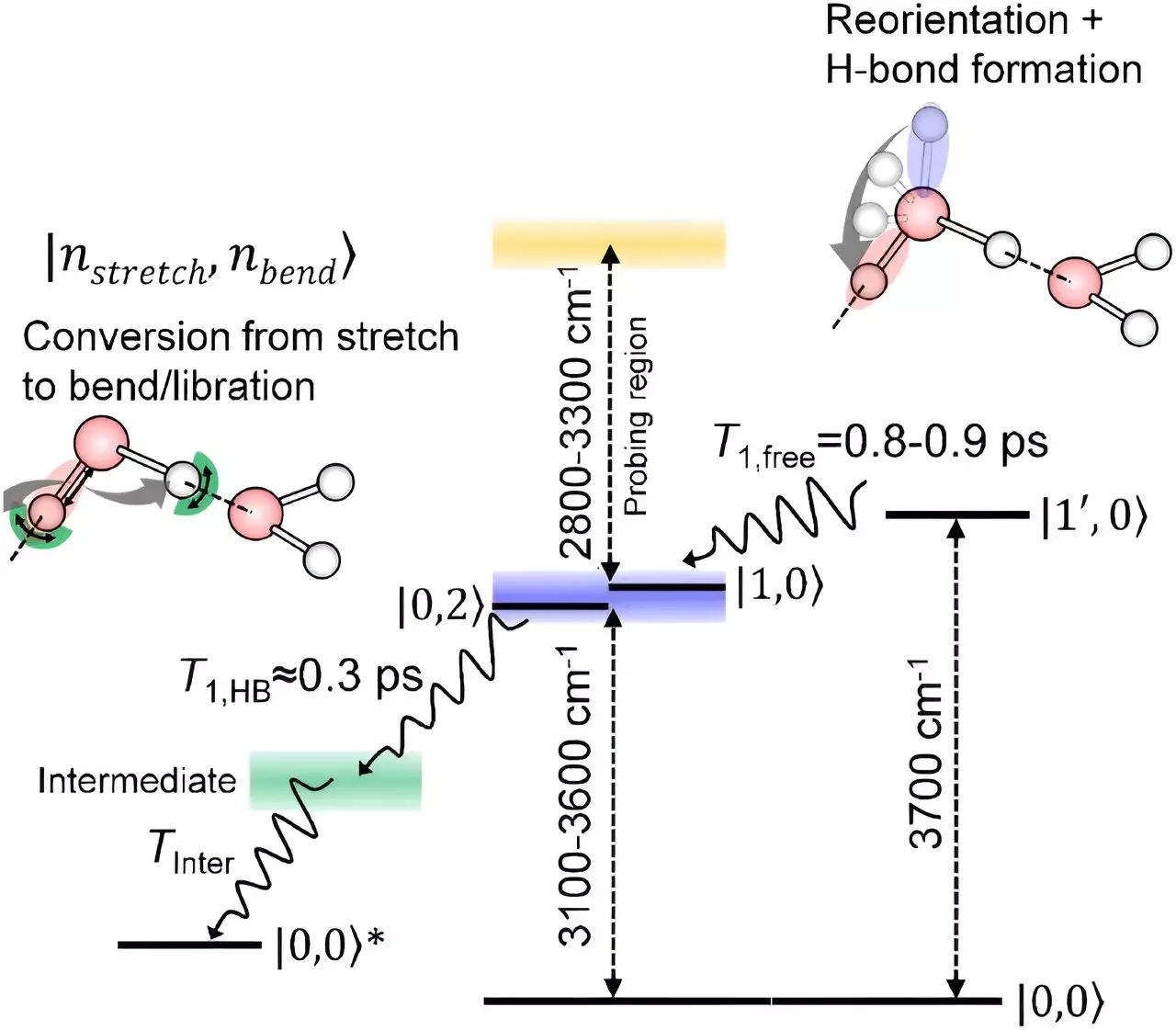
In recent years, researchers at the RIKEN Molecular Spectroscopy Laboratory, led by Tahei Tahara, have made significant strides in developing advanced spectroscopy techniques to study the behavior of water molecules at surfaces. Their innovative approach, based on infrared spectroscopy, has enabled them to detect the relaxation of oxygen-hydrogen bonds of surface water molecules. This breakthrough has provided valuable insights into how these bonds behave at the interface with air. The team’s findings have shed light on the mechanisms by which these surface molecules relax, offering a more complete picture of the behavior of water molecules at interfaces.
Through their spectroscopic technique, the researchers discovered that oxygen-hydrogen bonds of surface water molecules initially rotate without losing energy when exposed to the air. Subsequently, these bonds relax in a manner similar to the hydrogen-bond network observed in molecules within the bulk of the liquid. This observation suggests that molecules at the interface and those inside the liquid share a common relaxation process after interacting with neighboring molecules. The comprehensive picture painted by these findings provides a deeper understanding of how stretching of oxygen-hydrogen bonds relaxes at the surface of water.
With their newfound insights, Tahara and his team plan to further explore the applications of their spectroscopic technique in studying chemical reactions that take place at the interface of water. By delving into the interactions and dynamics of molecules at interfaces, the researchers aim to enhance our understanding of fundamental processes occurring in these systems. The implications of this research extend beyond the realm of water molecules, offering potential insights into broader scientific phenomena at interfaces.
The study of water molecules at interfaces presents a fascinating area of research with implications for diverse fields ranging from chemistry to materials science. The innovative spectroscopy techniques developed by researchers have opened new avenues for exploring the behavior of molecules at interfaces, providing valuable insights that contribute to our understanding of complex molecular interactions. Through continued research and exploration, scientists can unlock the mysteries of surface water molecules and gain a deeper appreciation for the intricate processes occurring at the interface of water.

Leave a Reply