Water oxidation using iridium oxide catalysts has shown great promise for green technologies, especially in clean energy processes. A recent study conducted by a team of researchers from SANKEN at Osaka University delved into the intricate workings of these catalysts, shedding light on the chemical species involved in the oxygen evolution reaction (OER). The research, published in the Journal of the American Chemical Society, utilized spectroscopy to examine how these chemical species interacted with the surrounding solution during the catalytic process.
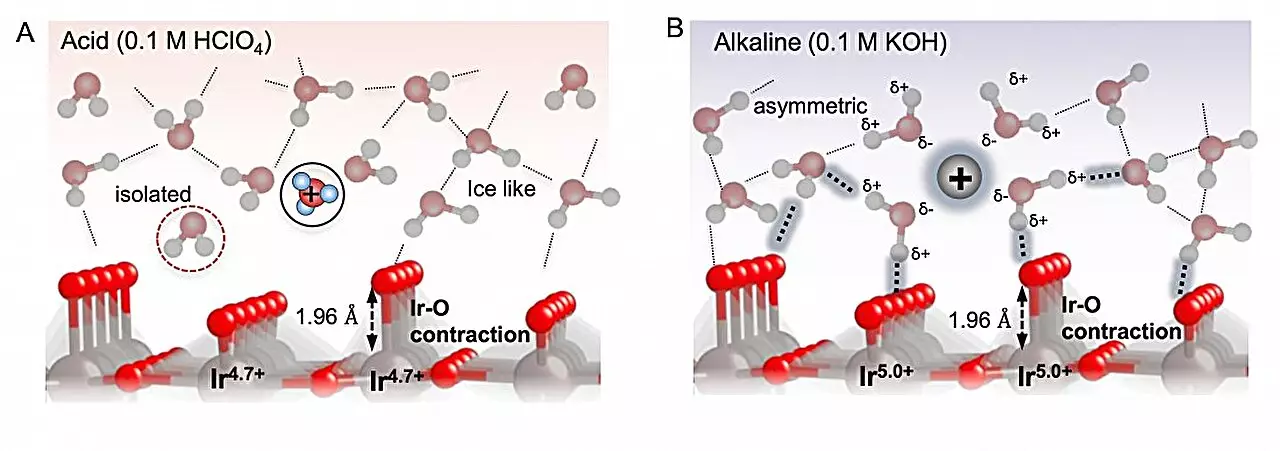
Catalytic processes can often be complex, involving multiple intermediate species on the path from the starting material to the desired product. To gain insight into these intermediates, operando techniques were employed, enabling real-time spectroscopic analysis of the reaction as it unfolds. By utilizing an electrode with an iridium oxide surface, the researchers investigated the oxidation of water molecules across solutions with varying pH levels.
One of the key findings of the study was the importance of long-range interactions between the intermediates in solution and the electrode surface. The binding of reaction intermediates to the electrode needed to strike a delicate balance – strong enough to allow interaction with the electrode, yet not so strong that they become immobilized. Surprisingly, the researchers discovered that the pH of the solution played a crucial role in influencing these interactions. In alkaline conditions, the proximity of water molecules to the electrode influenced the long-range interactions between the oxygenated species, ultimately affecting their binding to the surface.
Operando UV-Vis spectroscopy, X-ray absorption spectroscopy, and surface-enhanced infrared spectroscopy were instrumental in providing a direct look at the species involved in the catalytic process. This comprehensive approach allowed the researchers to go beyond simply examining electrode binding and gain a deeper understanding of catalyst performance. According to senior author Yu Katayama, this insight will be pivotal in optimizing the kinetics of the OER and enhancing the efficiency of water oxidation for green hydrogen production.
The implications of this research extend far beyond water oxidation alone. The findings from this study have the potential to contribute significantly to green hydrogen production, a key component of sustainable energy solutions. Furthermore, the integration of operando spectroscopy with complementary techniques opens up new possibilities for understanding the catalysis of various other processes, promising a more comprehensive approach to optimizing catalyst performance in the future.

Leave a Reply