In a groundbreaking study conducted by researchers at the University of Michigan, a new catalyst material called cobalt phthalocyanine has shown promising results in converting carbon dioxide into renewable fuels such as methanol. This innovation could potentially play a significant role in combating climate change by reducing greenhouse gas emissions and providing a sustainable source of clean energy.
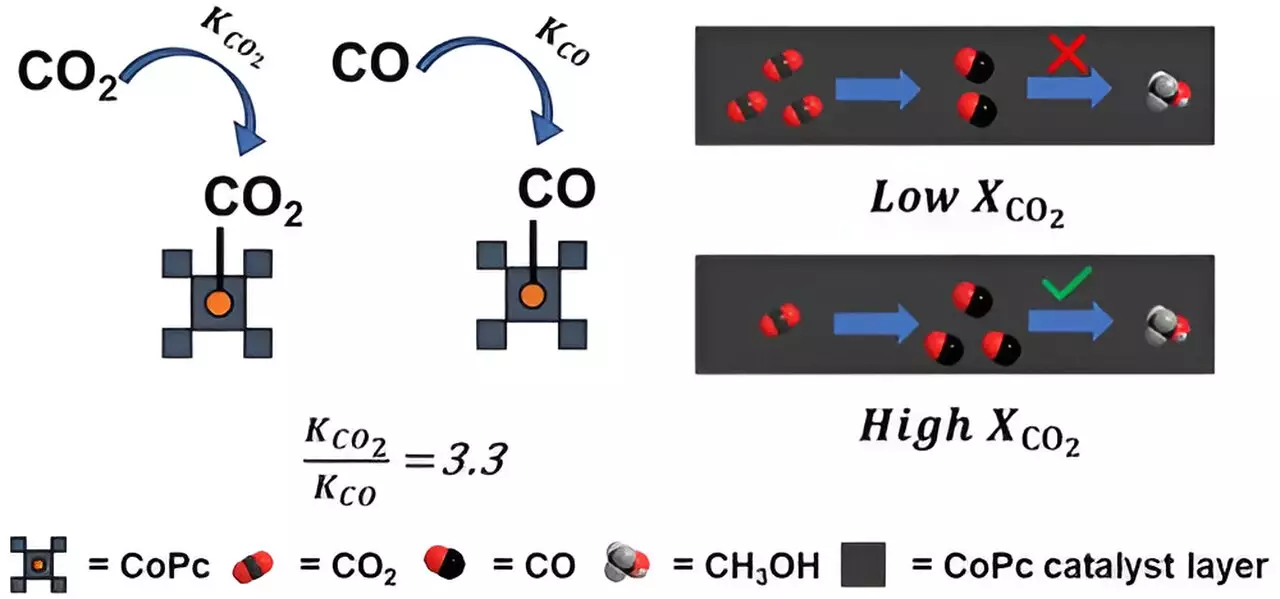
The process involves multiple reaction steps, with the first step converting carbon dioxide (CO2) into carbon monoxide (CO), followed by the conversion of CO into methanol. While the industrialization of converting CO2 into methanol has been achieved, doing so on a large scale through electrochemical processes has proven to be a challenge. Cobalt phthalocyanine acts as a molecular hook for CO2 or CO molecules, with the arrangement of these molecules around the cobalt metal playing a crucial role in determining how strongly each gas molecule binds.
One of the major limitations discovered by the researchers is that cobalt phthalocyanine binds much more strongly to CO2 molecules than to CO molecules. This results in CO being displaced by another CO2 molecule before it can be further converted into methanol. Advanced computational modeling and experimental measurements have confirmed that cobalt phthalocyanine binds CO2 over three times more tightly than it binds carbon monoxide, highlighting the need for a redesign of the catalyst’s binding affinity.
In order to overcome this roadblock, the researchers propose redesigning the cobalt phthalocyanine catalyst to enhance its interaction with CO and reduce its binding affinity to CO2. By addressing this issue, the door could be opened to efficiently convert CO2 waste into methanol fuel on a larger scale. This innovative approach brings together scientists and engineers to collectively brainstorm and gather insights, showcasing the importance of interdisciplinary collaboration in pushing the boundaries of scientific research.
The development of cobalt phthalocyanine as a catalyst for converting CO2 into renewable fuels represents a significant step forward in the quest for sustainable energy solutions. By unlocking the potential of this versatile material, researchers are paving the way for a greener future where carbon dioxide emissions can be harnessed and transformed into valuable resources. This study serves as a shining example of how innovation and collaboration can lead to impactful discoveries with far-reaching implications for the environment and society as a whole.

Leave a Reply