The world is currently facing a climate crisis, with carbon dioxide (CO2) emissions being a major contributor to global warming. However, researchers have found a potential solution to this problem in the form of a low-cost, tin-based catalyst that can convert CO2 into valuable chemicals such as ethanol, acetic acid, and formic acid. This breakthrough could revolutionize the way we approach carbon emissions and provide a sustainable path forward for industrial operations.
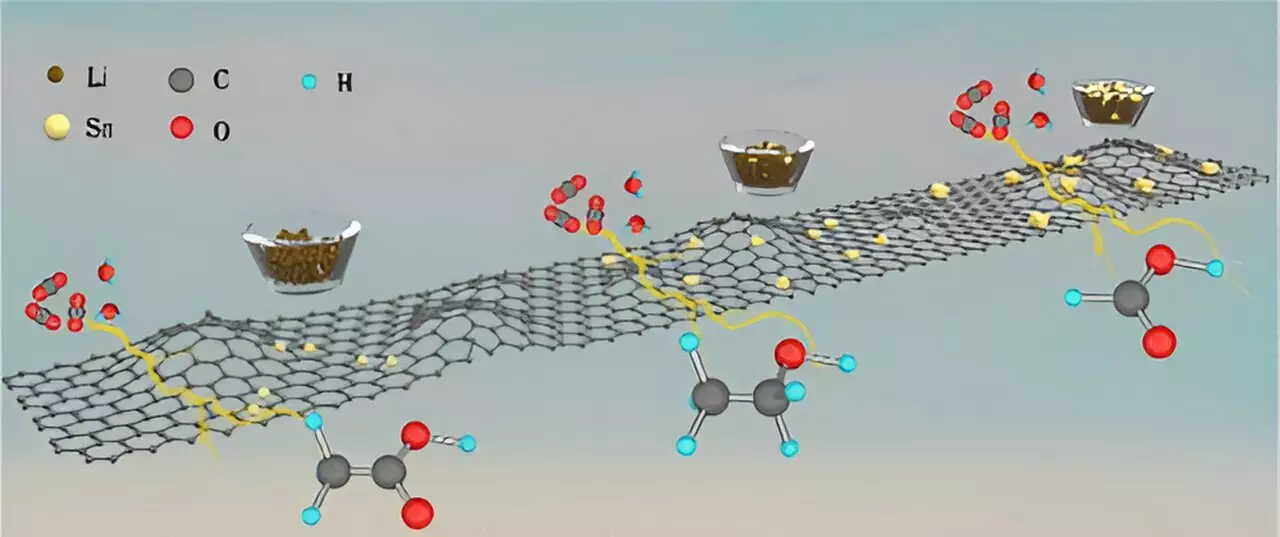
The catalysts developed by a team of scientists from the U.S. Department of Energy’s Argonne National Laboratory, Northern Illinois University, and Valparaiso University are based on tin metal deposited over a carbon support. These catalysts have shown remarkable efficiency in converting CO2 into ethanol, acetic acid, and formic acid, with selectivity rates of 90% or higher for each chemical. This level of control over the conversion process is unprecedented and holds great promise for the future of sustainable chemistry.
The Conversion Process
The team utilized electrocatalytic conversion, a method in which CO2 conversion over a catalyst is driven by electricity. By varying the size of tin used in the catalysts, ranging from single atoms to ultrasmall clusters to larger nano-crystallites, the researchers were able to control the production of specific chemicals. This innovative approach allowed for a high degree of selectivity in the conversion process, paving the way for more efficient and sustainable chemical production.
Through computational and experimental studies, the researchers uncovered several insights into the reaction mechanisms involved in the formation of ethanol, acetic acid, and formic acid. One particularly notable discovery was the observation of a changing reaction path when ordinary water was replaced with deuterated water in the conversion process. This phenomenon, known as the kinetic isotope effect, had never been observed in CO2 conversion before, highlighting the novel and groundbreaking nature of this research.
The ultimate goal of this research is to integrate the newly discovered catalysts into a low-temperature electrolyzer powered by locally generated wind and solar electricity. This would enable the production of desired chemicals for local consumption, reducing the need for CO2 transport and storage costs. The versatility and efficiency of this technology make it an ideal solution for addressing the challenges posed by carbon emissions and climate change.
The development of tin-based catalysts for CO2 conversion represents a significant step forward in the quest for sustainable chemical production. With further research and development, this technology has the potential to revolutionize the way we approach carbon emissions and create a more environmentally friendly future for generations to come.

Leave a Reply