Heterocyclic compounds, known for their versatile and excellent physiological activities, are highly valued in the chemical and pharmaceutical industries. However, the traditional methods of synthesizing these compounds often involve high temperature and pressure conditions or the use of precious metal catalysts, which add to both the economic and environmental costs. In a recent study published in the journal Advanced Synthesis & Catalysis, a team of researchers from Japan and Bangladesh proposed a simple and effective solution to overcome these challenges.
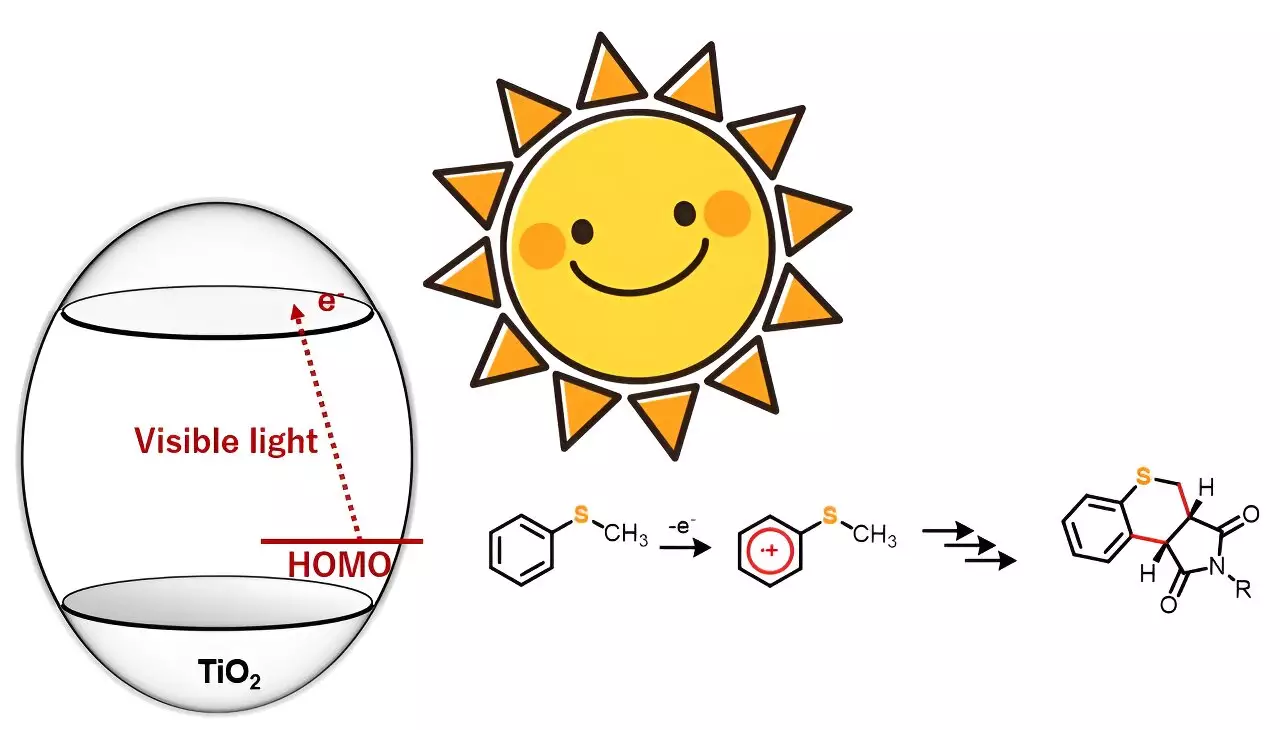
The study, led by Professor Yutaka Hitomi from Doshisha University, involved the synthesis of sulfur-containing heterocyclic compounds using a photocatalyst called titanium dioxide (TiO2) and visible light. Unlike previous studies that used ultraviolet light, the research team discovered that under anaerobic conditions, sulfur-containing organic compounds reacted with maleimide derivatives when exposed to blue light. This reaction led to the formation of a new heterocyclic compound with dual carbon–carbon bonds.
TiO2 has gained attention among synthetic chemists as a photocatalyst for driving organic reactions. However, many of these processes typically require ultraviolet light to trigger the reaction. In this study, the researchers found that visible light, specifically blue light, can be used to selectively oxidize the substrate molecules. This approach opens up possibilities for various organic chemical reactions.
To demonstrate the effectiveness of their approach, the researchers selected five 4-substituted thioanisoles and four N-substituted maleimides for the annulation or ring formation reactions. Initially, no reaction occurred when the starting materials were irradiated with blue light. However, upon introducing TiO2 into the reaction system, the team successfully synthesized 20 different thiochromenopyrroledione derivatives with moderate-to-high yield.
Insights into the Mechanistic Aspects
Through their experiments, the research team also observed the substituent effect in the reactions, providing valuable insights into the underlying mechanisms. They postulated that the charge transfer from thioanisole to the conduction band of TiO2 initiated the reaction. Blue light then triggered the one-electron oxidation of thioanisole, leading to the generation of α-thioalkyl radicals through deprotonation.
This refined approach not only demonstrated the potential of TiO2 for visible light photocatalysis but also uncovered crucial aspects of complex heterocyclic compound synthesis. By utilizing visible light and a readily available photocatalyst, this method offers a more energy-efficient and sustainable alternative to the resource-intensive industrial chemical processes in use today.
The findings of this study have significant implications for various industries, particularly in pharmaceutical synthesis. The wide adoption of visible light photocatalysis could revolutionize the accessible and affordable synthesis of pharmaceuticals, benefiting the health and well-being of millions of people worldwide.
Professor Hitomi and his team’s efforts have opened new avenues for organic synthesis, offering the potential to transform multiple chemical industries. The development of a sustainable chemical industry is a driving force behind this research, and this study represents a positive step in that direction.
The use of visible light photocatalysis, particularly with TiO2 as a photocatalyst, presents a promising approach to synthesizing heterocyclic compounds. By harnessing the power of visible light, researchers can reduce the environmental and economic costs associated with traditional methods. As the field of organic synthesis continues to advance, the potential for visible light photocatalysis to shape the future of chemical industries becomes increasingly evident.

Leave a Reply