With the increasing use of electric and hybrid vehicles worldwide, the development of battery technologies that are safe, high-performing, and scalable has become paramount. One promising avenue of research is the utilization of rechargeable multivalent metal batteries, which employ multivalent ions such as magnesium (Mg) and calcium (Ca) as anode materials. To achieve high energy densities, a combination of suitable anodes, cathodes, and electrolytes is required.
While researchers have made significant progress in identifying cost-effective anode materials for multivalent metal batteries, finding suitable electrolytes has proven to be more challenging. Many proposed electrolytes either rely on difficult-to-source materials or involve complex synthesis processes, making them unsuitable for large-scale production. As a result, the development of high-performance and affordable electrolyte systems has been hindered.
Recently, a team of researchers from Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology introduced a new and universal method to address the electrolyte challenge. Their findings, published in Nature Energy, offer a promising strategy for developing highly performing and scalable electrolytes for multivalent metal batteries.
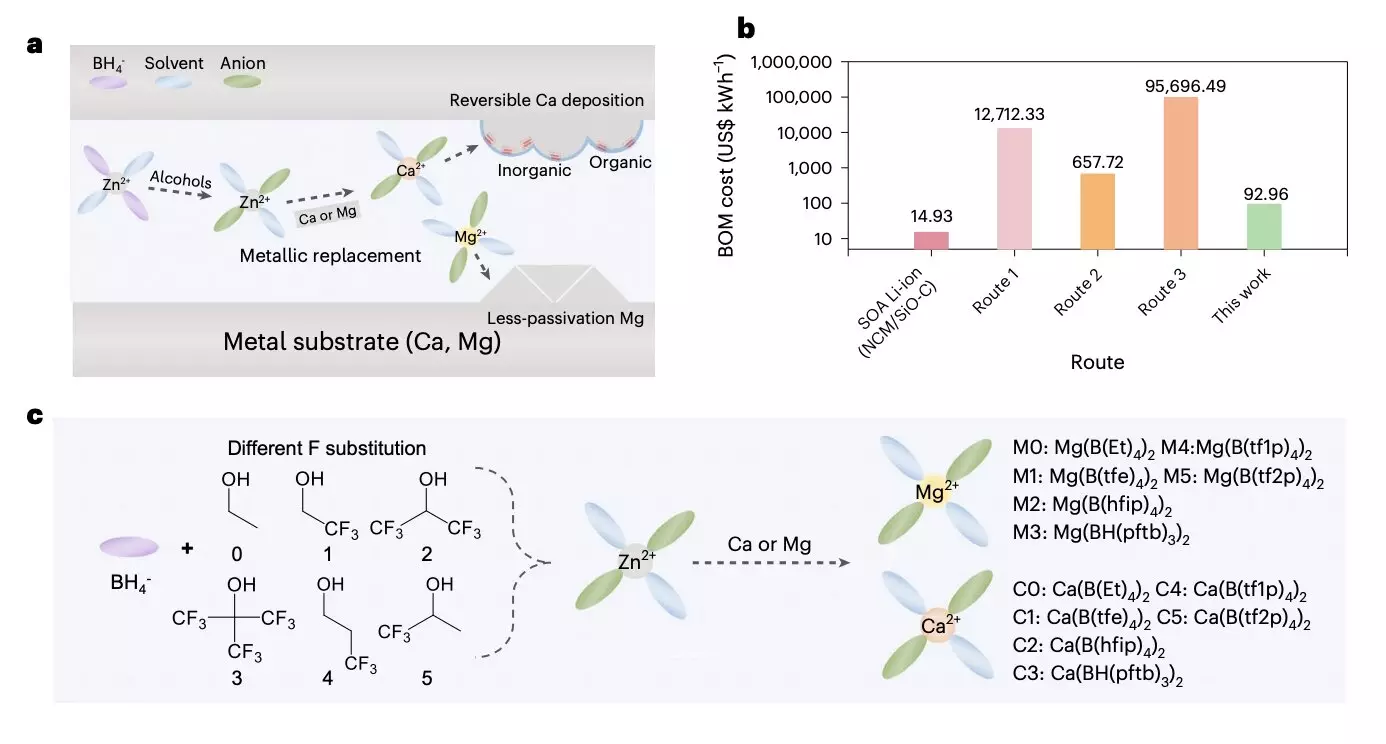
The researchers’ method involves several steps. First, they initiated a chemical reaction between a readily available Zn(BH4)2 precursor and various fluoroalcohols to produce target anions with different branched chains. These anion solvates were then reacted with low-cost metal foils possessing higher metal activity, resulting in the production of target solvation structures. To enhance battery cycling stability, the researchers proposed the formation of a passivation layer using two types of Ca solvates.
By carefully adjusting the chain length of the precursor and the degree of F-substitution, the researchers were able to fine-tune the participation of anions in the primary solvation shell. Their study demonstrated that a completely dissociated Mg organoborate electrolyte enabled high current endurance and enhanced electrochemical kinetics. On the other hand, the Ca organoborate electrolyte with strong coordination and B-H inclusion offered a stable solid-electrolyte interphase with high coulombic efficiency.
Using their method, the research team successfully created a high-loading battery prototype based on Mg/S. This prototype, which featured a 30 μm Mg anode, a low electrolyte/sulfur ratio, and a modified separator/interlayer, achieved a promising energy density of 53.4 Wh kg-1 in initial tests. These results underscore the potential of the approach to produce favorable and cost-effective electrolytes for multivalent metal batteries.
The method introduced in this study holds great promise for the development of various reversible electrolyte systems that rely on affordable materials and simpler processing strategies. By overcoming the challenges associated with electrolytes, researchers can pave the way for the creation of scalable and safe multivalent metal batteries with even higher energy densities. This research represents a significant step forward in the quest for safer and more affordable battery technologies, helping to drive the widespread adoption of electric and hybrid vehicles worldwide.
As the demand for electric vehicles increases, there is a pressing need for safer and more efficient battery technologies. The development of reliable electrolytes is a critical component in achieving this goal. The research conducted by the team at Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology offers a revolutionary approach to electrolyte development for multivalent metal batteries. By utilizing a universal cation replacement method, the researchers were able to produce highly performing and scalable electrolytes derived from cost-effective materials. This breakthrough could pave the way for the creation of safer and more affordable battery technologies, ultimately driving the transition towards a greener transportation future.

Leave a Reply