Protein folding is a crucial process in cellular biology, ensuring that proteins adopt their correct three-dimensional structures to function properly. With the help of cryo-electron tomography (cryo-ET), researchers are now able to gain unprecedented insights into how proteins fold within cells. A recent study conducted by the MPI of Biochemistry in Martinsried and the University Medical Center Göttingen has shed light on the intricate mechanisms of protein folding helpers known as chaperonin complexes in the bacterium E. coli.
Chaperonins, like the GroEL and GroES complexes found in bacteria, play a vital role in guiding newly synthesized proteins to fold accurately. These chaperonins encapsulate proteins within their barrel-like structures, shielding them from the cellular environment. Understanding how these chaperonin complexes function is essential, as misfolded proteins can lead to various diseases such as Alzheimer’s and Parkinson’s.
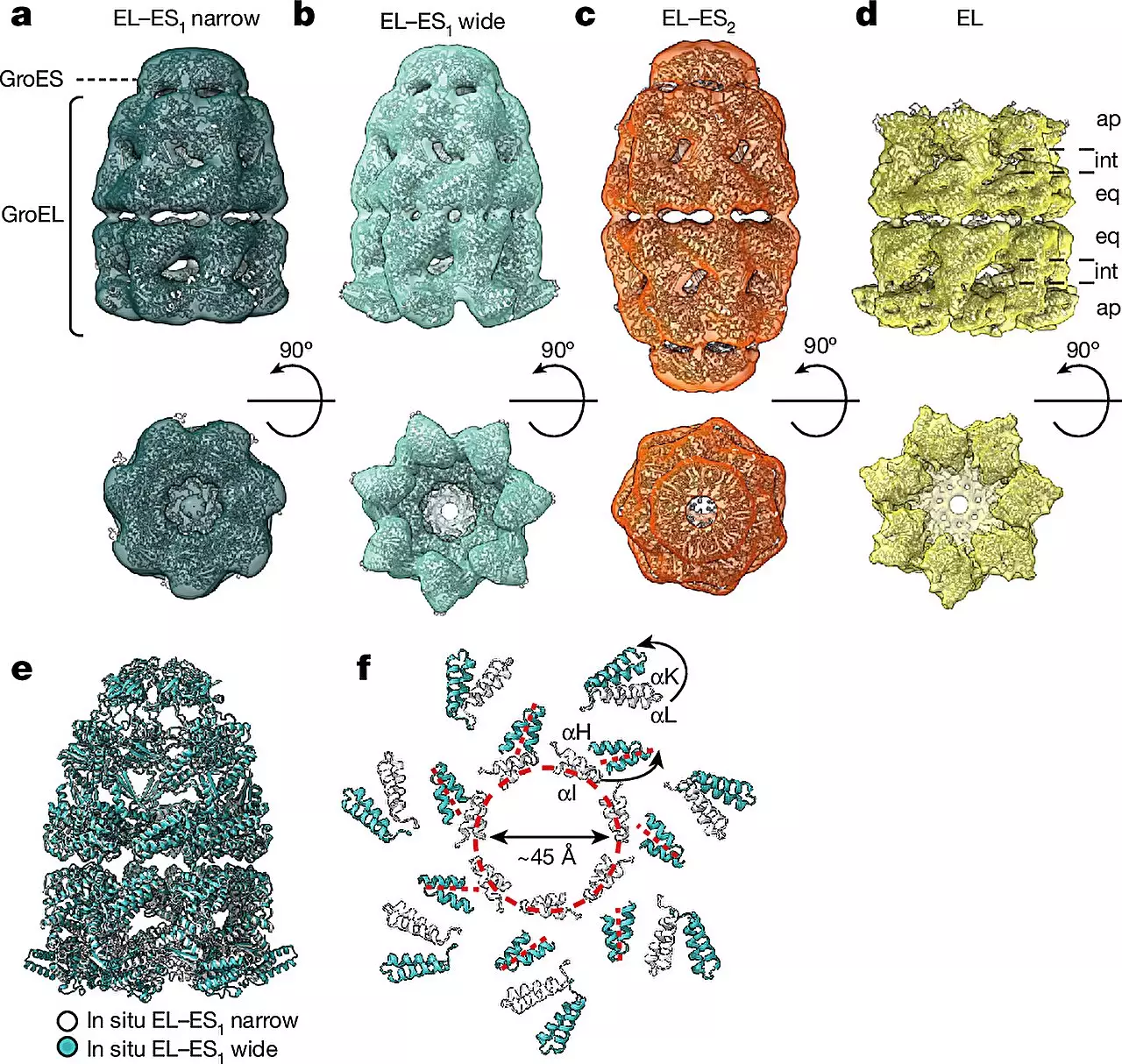
By combining cryo-ET with single-particle cryo-electron microscopy (cryo-EM) and quantitative mass spectrometry, researchers were able to observe the conformational changes in chaperonin complexes within living cells. This approach allowed them to identify two main forms of the GroEL-GroES complex – the “bullet” and “football” shapes. These shapes differ in their structural symmetry, providing new insights into the dynamics of chaperonin-assisted protein folding.
Decades of experiments with purified chaperonin complexes in test tubes have yielded conflicting results on their mechanism of action. The introduction of cellular cryo-ET has allowed researchers to visualize these complexes directly within the native cellular environment, providing a more accurate representation of how they function in vivo. This approach has revealed that chaperonins alternate between the bullet and football shapes during the protein folding process, highlighting the complexity of their assembly dynamics.
The detailed insights obtained from cryo-ET studies on chaperonin complexes hold significant implications for disease treatment. By understanding how these complexes facilitate proper protein folding, researchers may be able to develop novel strategies for tackling protein misfolding diseases. This research opens up new possibilities for therapeutic interventions targeting the molecular machinery involved in protein folding processes.
Cryo-electron tomography represents a groundbreaking technology that has revolutionized the field of protein research. The ability to visualize protein folding in living cells at high resolution provides a deeper understanding of the molecular mechanisms underlying cellular processes. The study conducted by the MPI of Biochemistry and the University Medical Center Göttingen exemplifies the power of cryo-ET in unraveling the complexities of protein folding helpers, paving the way for future discoveries in the field of structural biology.

Leave a Reply