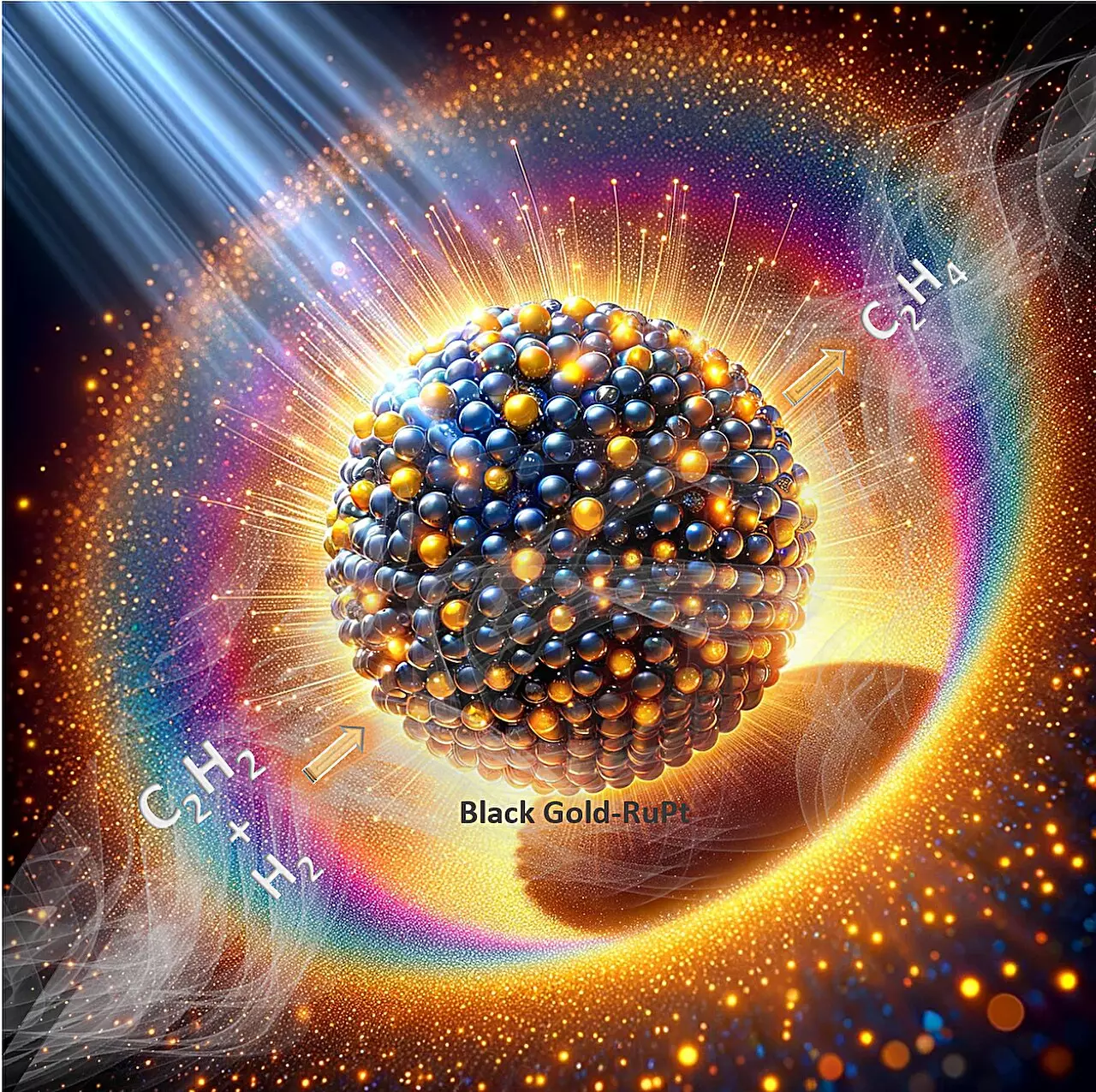
In a groundbreaking development, Prof. Polshettiwar’s research group at Tata Institute of Fundamental Research (TIFR), Mumbai has successfully created a revolutionary “plasmonic reduction catalyst stable in air.” This remarkable catalyst defies the common instability issues faced by reduction catalysts when exposed to air. By combining platinum-doped ruthenium clusters with “plasmonic black gold,” the catalyst harnesses visible light efficiently and generates multiple hot spots through plasmonic coupling, significantly enhancing its catalytic performance. The findings of this innovative work were published in the prestigious journal Nature Communications.
One of the key distinguishing factors of this catalyst is its exceptional performance in the semi-hydrogenation of acetylene, a crucial industrial process. Surprisingly, the catalyst reaches its peak efficiency in the presence of both air and reactants. Under visible light illumination, without the need for external heating, the catalyst achieves an astounding ethene production rate of 320 mmol g−1 h−1 with an impressive selectivity of approximately 90%. These results surpass the performance of all previously known plasmonic and traditional thermal catalysts. To ensure utmost stability, this exceptional catalyst maintained its efficiency for a remarkable duration of at least 100 hours.
Through meticulous research, the team discovered that plasmon-mediated simultaneous reduction and oxidation processes at the active sites during the reaction contribute to the unparalleled stability of the catalyst. Additionally, finite-difference time-domain (FDTD) simulations revealed a five-fold increase in the electric field compared to the original catalyst. This significant field enhancement, resulting from the near-field coupling between the RuPt nanoparticles and DPC, plays a vital role in activating chemical bonds, thereby boosting the catalytic activity.
The catalyst’s effectiveness is evident through its kinetic isotope effect (KIE), which is larger under light than in darkness at all temperatures. This observation suggests the influential role of non-thermal effects alongside the photothermal activation of the reactants. Profound insights into the reaction mechanism over the oxide surface were obtained through in-situ DRIFTS and DFT studies. These studies highlighted the crucial role of intermediates in selectivity. The partially oxidized RuPt catalyst surface produces di-σ-bonded acetylene, which then undergoes several transformative steps to yield ethene.
Undoubtedly, the findings of Prof. Polshettiwar’s research group offer unprecedented contributions to the field of plasmonic catalysis. By providing vital insights into the simultaneous reduction and oxidation processes, this breakthrough paves the way for the development of sustainable and energy-efficient catalytic systems. The unique stability and remarkable efficiency achieved by this novel plasmonic reduction catalyst mark a turning point in catalytic science. With a better understanding of the catalyst’s behavior and the crucial role of intermediates, scientists can now advance their efforts towards more effective and eco-friendly catalytic systems. The potential impact of this research on various industries, including the chemical, pharmaceutical, and energy sectors, is truly groundbreaking, promising a future of enhanced efficiency and sustainability.

Leave a Reply