Perfluoroalkyl substances (PFAS), often referred to as “forever chemicals,” have emerged as a significant environmental and health concern due to their persistent nature and harmful effects. Initially recognized for their stability and resistance to water and heat, PFAS have found their way into various products, leading to widespread contamination in water, soil, and even human bodies. Despite efforts to phase out PFAS production, their long lifespan and resistance to decomposition pose a challenge for effective treatment and disposal.
The Breakthrough Research
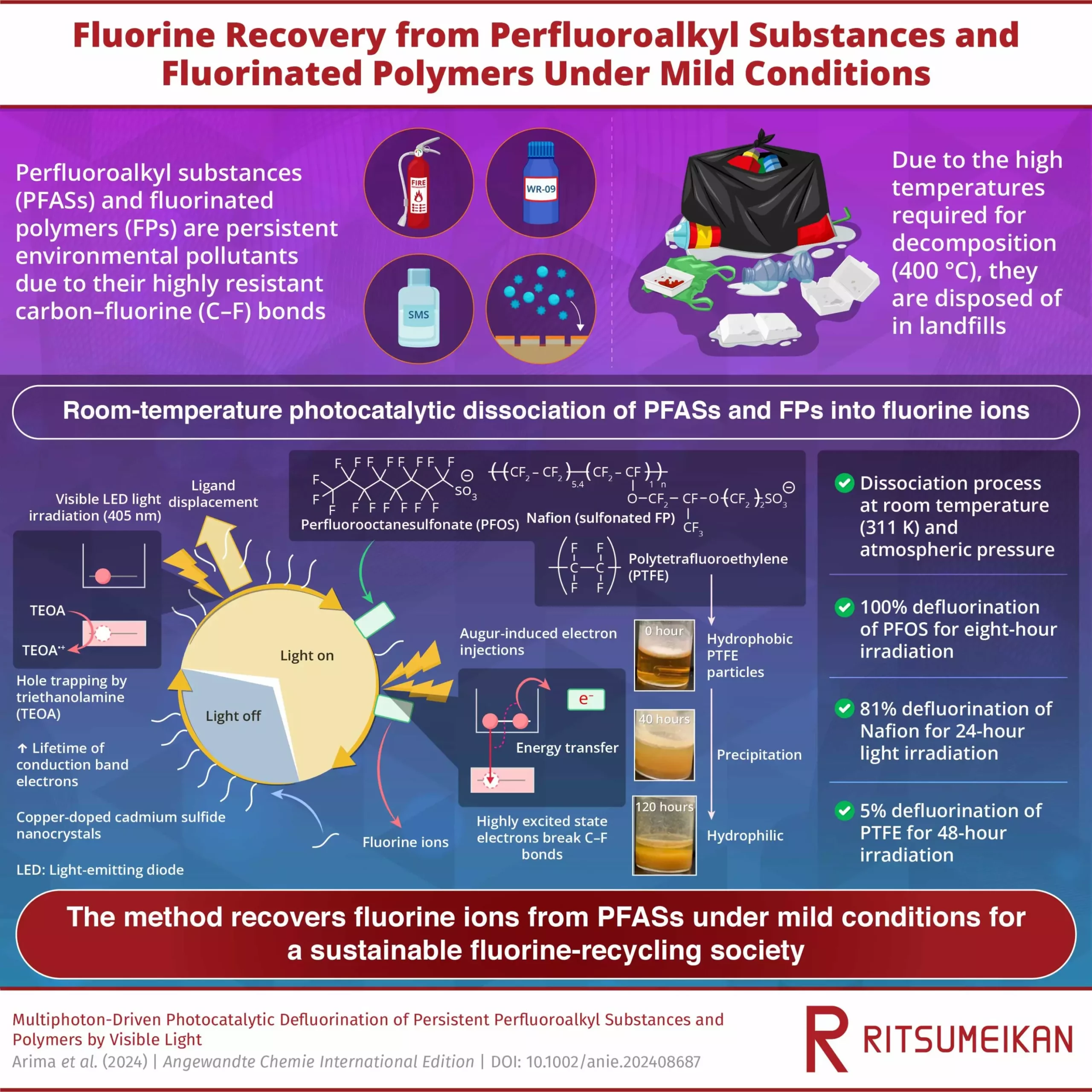
A recent study conducted by researchers at Ritsumeikan University introduces a room-temperature defluorination method that has the potential to revolutionize PFAS treatment. Published in the journal Angewandte Chemie International Edition, the study outlines a photocatalytic process that utilizes visible light to break down PFAS and other fluorinated polymers at room temperature. This groundbreaking method aims to address the shortcomings of conventional treatment techniques and contribute to the development of a sustainable fluorine-recycling society.
The proposed method involves irradiating visible LED light onto semiconductor nanocrystals, specifically cadmium sulfide (CdS) and copper-doped CdS (Cu-CdS) nanocrystals with surface ligands of mercaptopropionic acid (MPA). By introducing PFAS, fluorinated polymers (FPs), and triethanolamine (TEOA) to the solution, the researchers were able to generate electrons with a high reduction potential that effectively break down the carbon-fluorine bonds in PFAS molecules. This innovative approach resulted in 100% defluorination of perfluorooctanesulfonate (PFOS) within just 8 hours of light exposure, showcasing the effectiveness of the method.
During the photocatalytic reaction, the CdS nanocrystals and TEOA work synergistically to capture photoexcited electrons and prevent them from recombining with holes, thus extending the lifetime of reactive electrons available for PFAS decomposition. The highly excited electrons generated through Auger recombination exhibit sufficient energy to engage in chemical reactions with PFAS molecules adsorbed on the nanocrystal surface, resulting in the cleavage of carbon-fluorine bonds and the removal of fluorine ions. The efficiency of defluorination was found to increase with the amount of nanocrystals and TEOA used, as well as the duration of light exposure, highlighting the versatility and scalability of the method.
Implications for Sustainability
By enabling the recovery of fluorine from waste PFAS and fluorinated polymers, the room-temperature defluorination method offers a promising solution for reducing dependence on fluorine production and promoting sustainable recycling practices. The ability to achieve high defluorination rates for PFAS and FPs, including Nafion, a widely used fluoropolymer, opens up opportunities for the development of recycling technologies across various industries. This breakthrough research not only addresses the pressing issue of PFAS contamination but also contributes to the advancement of recycling technologies and the establishment of a more sustainable society.
The room-temperature defluorination method developed by researchers at Ritsumeikan University represents a significant advancement in PFAS treatment and recycling. By harnessing the power of photocatalysis and visible light, this innovative approach offers a safe, effective, and sustainable solution for addressing the challenges posed by “forever chemicals.” As we continue to explore new strategies for environmental remediation and resource recovery, the room-temperature defluorination method stands out as a beacon of hope in the quest for a cleaner and healthier planet.

Leave a Reply