In a groundbreaking research effort, a team of scientists from Pohang University of Science and Technology (POSTECH), the Korea Research Institute of Chemical Technology, and Chonnam National University have devised a method for effectively separating well-mixed mixtures. This technique, developed by Professor Jee-hoon Han from the Department of Chemical Engineering at POSTECH, aims to streamline the synthesis and purification of ionic liquids, a type of salt that remains in liquid form at room temperature or even lower temperatures due to the strong electrical interactions between their ions.
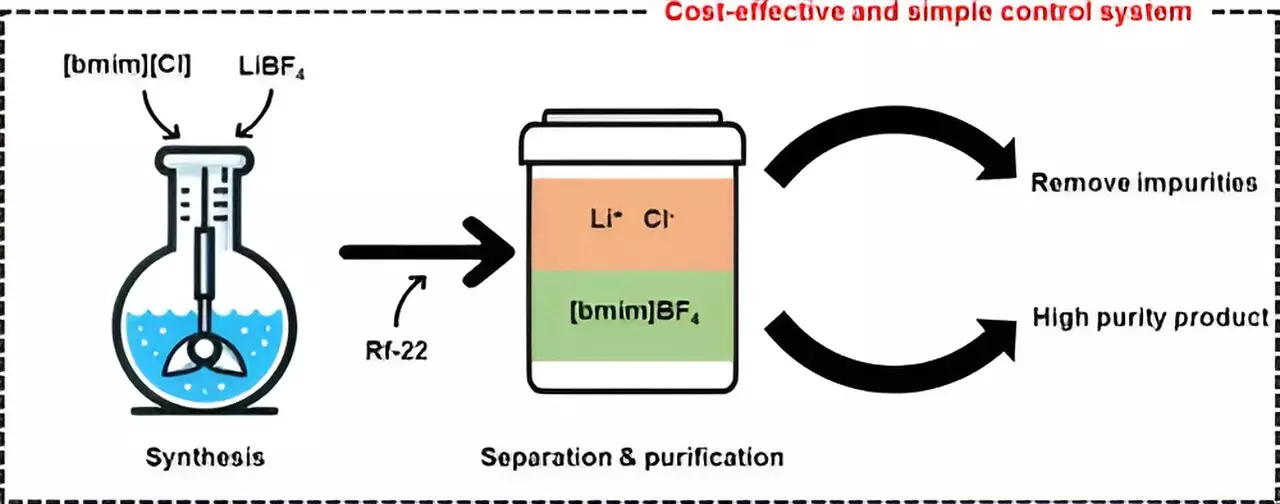
Ionic liquids are gaining increasing attention due to their unique properties such as nonflammability, low volatility, and thermal and chemical stability, which make them valuable for a range of industrial applications including catalysts and electrolytes. One of the most extensively studied ionic liquids is [bmim][BF4], known for its high stability and low toxicity. However, the arduous and costly process of removing impurities like lithium chloride (LiCl) during synthesis has posed a major obstacle to the commercialization of this technology.
In their study, the researchers employed halocarbon refrigerants, specifically chlorodifluoromethane (Rf-22), to synthesize the ionic liquid [bmim][BF4] in a more cost-effective and efficient manner compared to traditional methods. By utilizing Rf-22 as a phase separation mediator, they were able to induce the mixture containing methylimidazole to separate into distinct layers, akin to the separation of oil and water. Through varying the ratios of [bmim][BF4], water, and halocarbon mixtures, the team observed phase separation and constructed a ternary phase diagram model to visualize the composition and phases of the mixture containing the three components.
Through the application of the ternary phase diagram model, the researchers successfully produced high-purity [bmim][BF4] with a purity exceeding 99%. Additionally, they efficiently recovered and recycled the layer containing methylimidazole, which was not involved in the synthesis reaction. Furthermore, the team conducted process simulations to assess the economic viability of the purification technology developed in this study. Based on a cost analysis for producing 1 ton of [bmim][BF4] per day, they established that the minimum selling price would be approximately $12,000 per ton, making it a more competitive option compared to existing process technologies and highlighting the potential for commercialization of this innovative technique.
The novel technique developed by Professor Jee-hoon Han and his research team offers a promising solution for the efficient synthesis and purification of ionic liquids, particularly [bmim][BF4]. By leveraging halocarbon refrigerants and employing a ternary phase diagram model, the researchers were able to achieve high purity levels and economic feasibility in their process. This breakthrough paves the way for the widespread adoption and commercialization of this technology, opening up new possibilities for various industrial applications of ionic liquids.

Leave a Reply