The research conducted by McGill University on using copper nanoclusters as a catalyst to convert carbon dioxide into methane marks a significant breakthrough in the field of clean energy production. Unlike traditional methods that contribute to the increase of CO2 in the atmosphere, this new electrocatalysis process offers a sustainable solution to combat climate change.
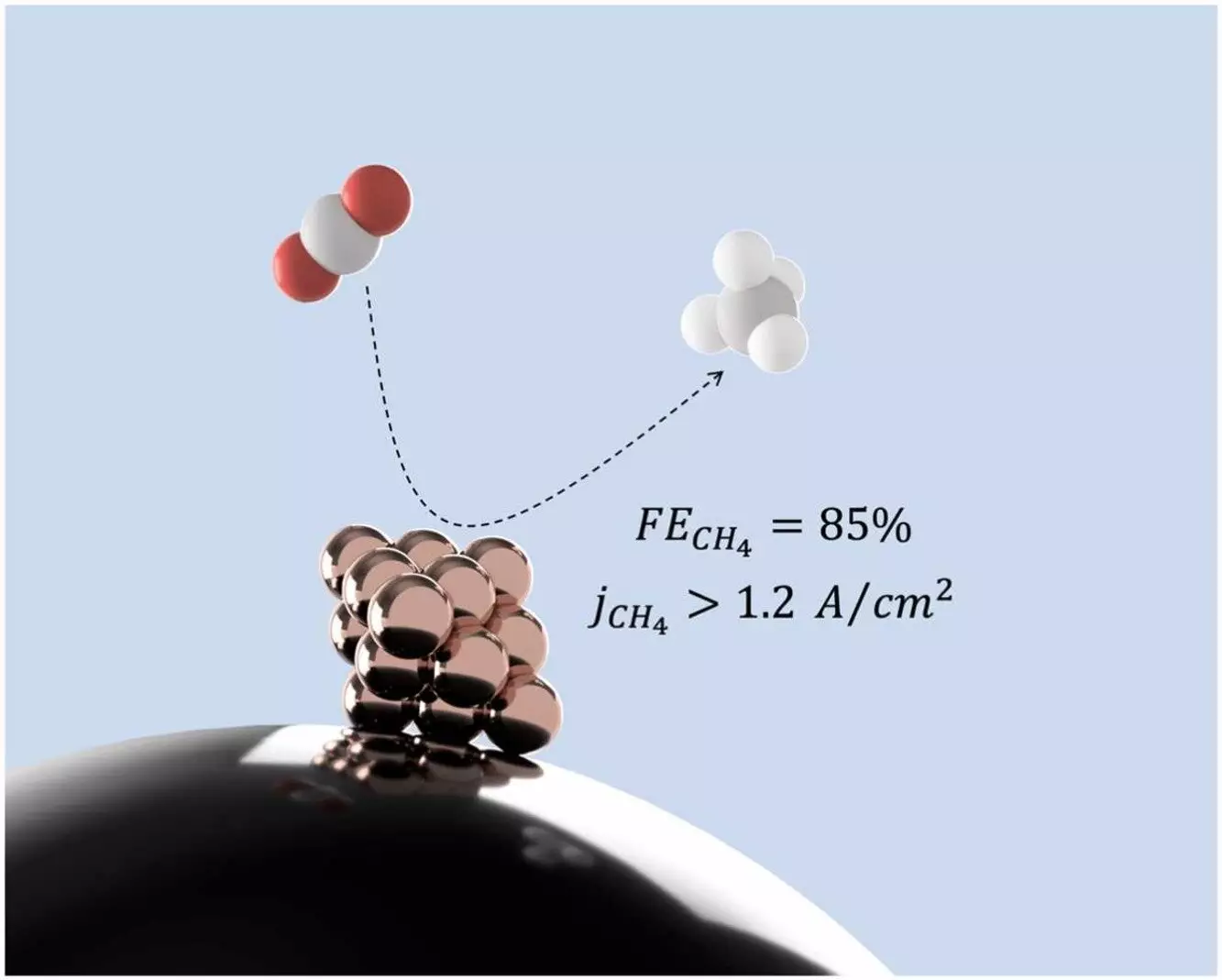
According to Mahdi Salehi, a Ph.D. candidate at McGill University, the use of copper nanoclusters has the potential to transform carbon dioxide from the atmosphere into methane, which can then be utilized as a clean energy source. By capturing and recycling any carbon dioxide released during the combustion of methane, a closed “carbon loop” is created, effectively reducing the emission of new CO2 into the atmosphere.
The research, recently published in the journal Applied Catalysis B: Environment and Energy, showcased the effectiveness of small copper nanoclusters in producing methane. Salehi emphasized that the size and structure of the nanoclusters play a crucial role in determining the outcome of the conversion reaction. This discovery opens up new possibilities for advancing clean energy technologies.
Moving forward, the research team at McGill University plans to further refine the catalyst to improve its efficiency and explore its potential applications on a larger scale in industrial settings. By optimizing the catalytic process, they aim to pave the way for the widespread adoption of clean and sustainable energy sources. The findings from this study offer hope for a future where renewable energy production is both efficient and environmentally friendly.
The research conducted by McGill University highlights the promising role of copper nanoclusters in converting carbon dioxide into methane for clean energy production. By leveraging nanotechnology and electrocatalytic processes, this innovative approach presents a viable solution to reduce greenhouse gas emissions and combat climate change.

Leave a Reply