Understanding the intricate relationship between glycans (sugar chains) and key enzymes has significant implications for disease development and progression. Recent research has shed light on how a structure in glycans interacts with enzymes, contributing to a range of diseases. Glycans play a crucial role in physiological processes such as cell recognition, cell signaling, immune response, and protein folding. Hence, any alteration in glycan structure can lead to severe health conditions ranging from cancer to Alzheimer’s. It is imperative to delve deeper into how enzymes regulate glycan attachment to proteins and lipids to develop targeted therapeutic interventions.
The field of glycobiology focuses on understanding the production and function of glycans in physiological processes. Despite significant advancement in identifying enzymes responsible for glycan production in humans, there is a need to predict and fine-tune glycan structures within cells accurately. Dysregulated glycosylation processes are linked to various diseases, underscoring the importance of understanding enzyme activity in glycan attachment. The discovery of a structure in glycans called LacdiNAc (LDN) and its association with crucial bodily processes and disease development highlights the complexity of glycan-enzyme interactions.
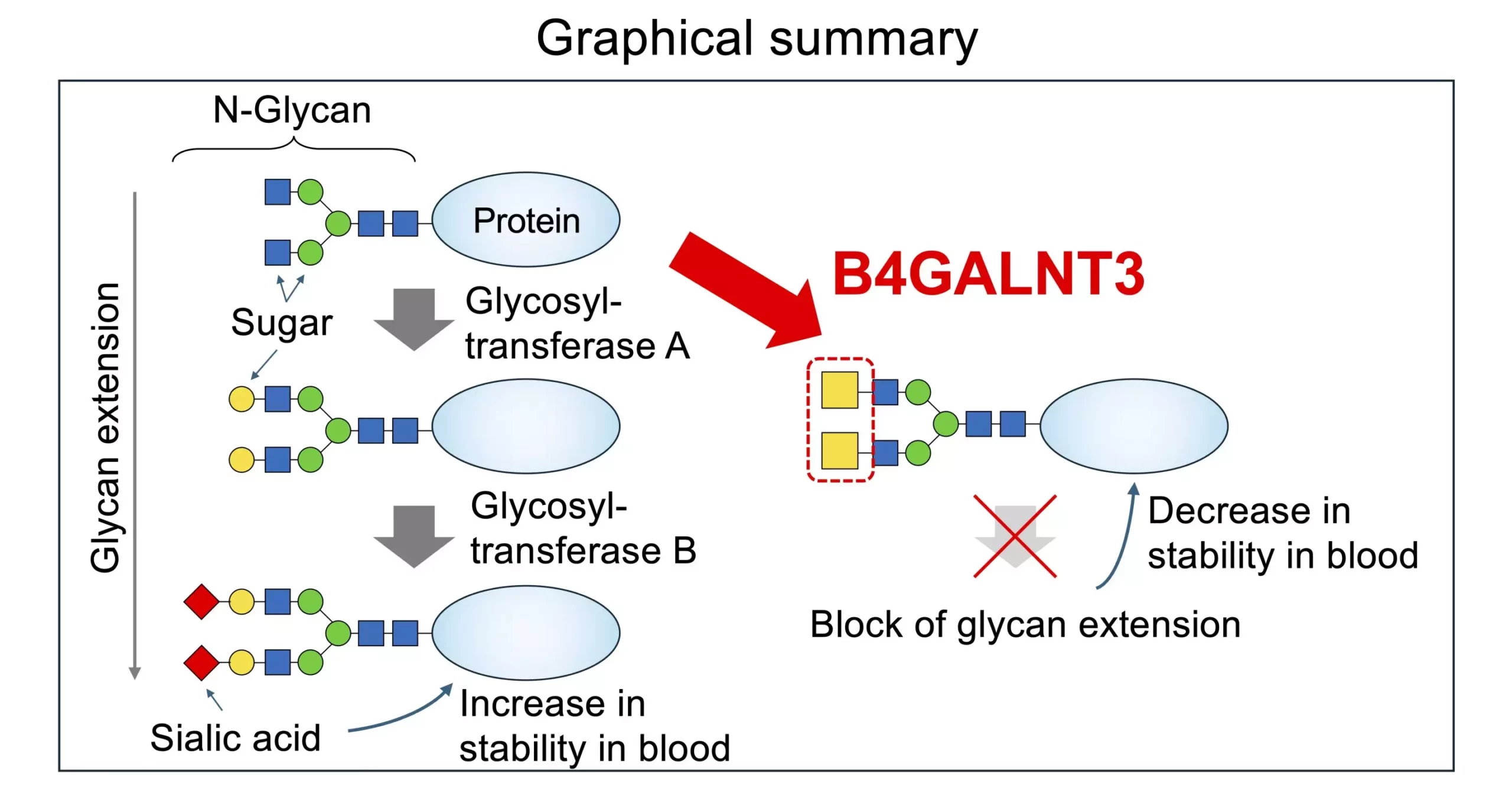
The biosynthesis of the LDN structure is catalyzed by the B4GALNT3 enzyme, which plays a pivotal role in modifying hormones and maintaining stem cells in the body. Variants of B4GALNT3 have been associated with lower bone density, higher fracture risk, and increased susceptibility to colon cancer when enzyme activity is compromised. Understanding how B4GALNT3 synthesizes LDN is crucial for unraveling disease pathogenesis. Recent advancements in structural biology, enzymology, and glycomics have provided researchers with tools to investigate enzyme activity and structural features influencing glycan modification.
Researchers discovered that B4GALNT3 contains a unique feature, the PA14 domain, which is essential for enzyme activity. Mutated versions of B4GALNT3 lacking the PA14 domain exhibited significantly decreased activity related to N-linked and O-linked glycans, highlighting the critical role of this domain in glycan synthesis. The presence of LDN in glycans negatively impacted the actions of glycosyltransferases in modifying or adding sugar residues, leading to altered glycoprotein structure and function. This finding emphasizes the distinct impact of LDN compared to other glycan structures like LacNAc.
Understanding the interplay between B4GALNT3, LDN, and enzyme activity opens new avenues for investigating disease mechanisms and identifying potential therapeutic targets. Further research is needed to elucidate the specific role of B4GALNT3 and LDN in disease pathogenesis and develop targeted interventions to modulate enzyme activity for therapeutic purposes. By unraveling the complex interactions between glycans and enzymes, researchers aim to pave the way for precision medicine approaches in treating diseases associated with glycan dysregulation.

Leave a Reply