The evolution of energy storage technology hinges significantly on the performance of solid-state batteries, where lithium and sodium metal anodes play a pivotal role. These highly reactive alkali metals have garnered attention for their potential to enhance battery efficiency, safety, and longevity. However, a profound understanding of their microstructure remains a necessity for optimizing their electrochemical properties. Recent pioneering research, led by Justus Liebig University Giessen (JLU) in collaboration with international teams from the U.S. and Canada, has illuminated opportunities for transforming the development of batteries by elucidating the microstructural characteristics of these metals.
For the first time, researchers harnessed an innovative technique to analyze the internal structure of alkali metals, particularly when deposited in a battery setting. Traditionally, the microstructure of metals—extending from the nanometer to micrometer scale—has shown a significant correlation with their electrochemical properties. While extensive research has been done on various metals, lithium and sodium have posed challenges mainly due to their high reactivity and tendency to form passive layers on surfaces, obscuring detailed analysis.
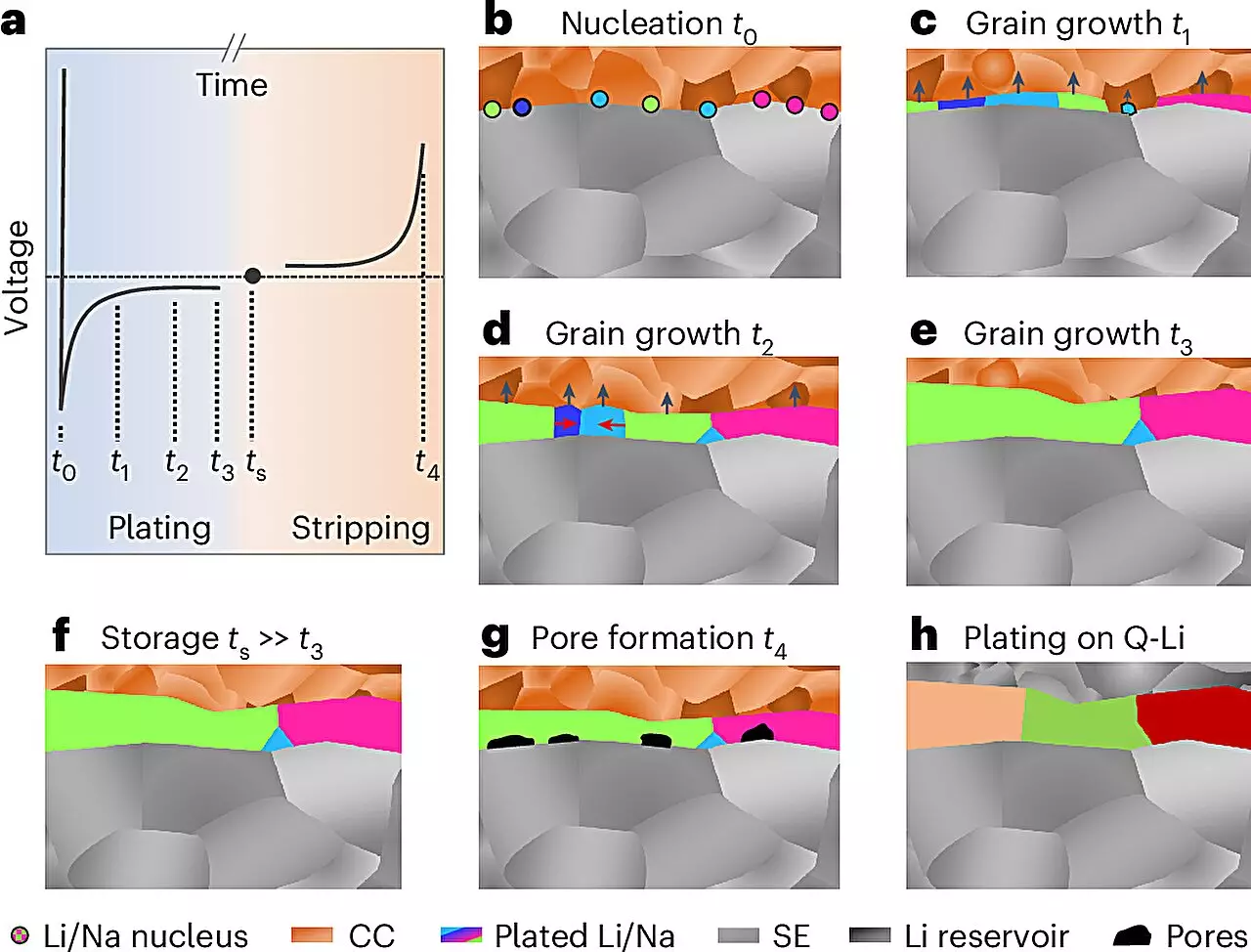
The collaborative effort led by Prof. Dr. Jürgen Janek at JLU employed a novel preparation and analysis protocol conducted at low temperatures and in inert environments. This sequence culminated in the application of electron backscatter diffraction to assess the local structures of electrochemically deposited lithium and sodium. This methodological advancement signifies a crucial leap forward, allowing for layers up to 100 micrometers thick to be studied with unprecedented clarity.
The novel findings regarding the grain size of the resulting lithium and sodium layers were unexpected and elucidated new growth mechanisms in these metal electrodes. Prof. Janek expressed excitement at the implications of these insights for future battery research, particularly emphasizing their relevance in exploring sodium battery systems, as underscored by the ongoing work within the POLiS (Post Lithium Energy Storage) excellence cluster.
Understanding how these metals grow and evolve during deposition is vital for enhancing their performance in batteries. The precision afforded by the new analysis method enables researchers to tailor microstructures intentionally to achieve desired characteristics, potentially steering the design of next-generation batteries.
Despite these advancements, integrating lithium and sodium metal anodes into practical battery systems remains fraught with challenges. Key hurdles include the tendency of these metals to undergo deformation during electrochemical cycling. This deformation manifests as pore formation during discharge and the growth of detrimental dendrites during charging, which can precipitate dangerous short circuits.
To counteract these issues, innovative solid-state electrolytes are being explored. The hope is that these ceramic materials could stabilize metal electrodes under operational conditions, allowing lithium and sodium to participate in more controlled electrochemical reactions. Such developments are critical as the energy storage landscape shifts toward solid-state solutions capable of matching or exceeding the performance of traditional lithium-ion batteries.
The pursuit of efficient solid-state batteries has galvanized research initiatives worldwide over the past decade. Spurred on by the work at JLU, collaboration with research teams from the University of California, Santa Barbara, and the University of Waterloo has yielded fruitful outcomes, underlining the potency of interdisciplinary partnerships in overcoming complex scientific challenges. The united efforts have ensured access to state-of-the-art materials research facilities, essential for conducting meticulous studies.
Prof. Janek emphasized the significance of collaborative expertise for successfully imaging lithium and sodium microstructures. Prior to this research, obtaining detailed images of these reactive metals proved difficult and had been accomplished only in limited cases. The achievement of imaging cross-sections of electrodes represents a paradigm shift that could unlock new pathways for the effective use of alkali metals in energy storage applications.
The research conducted at JLU and its collaborators marks a critical milestone in the journey toward high-performance solid-state batteries. The insights gleaned from studying the microstructures of lithium and sodium metal anodes could revolutionize our approach to energy storage technology. As researchers continue to refine methodologies and address the inherent challenges, the promise of safe, powerful, and long-lasting batteries draws ever closer, paving the way for a more sustainable energy future. The work is a compelling reminder of the importance of scientific exploration and collaborative synergy in addressing the world’s energy storage needs.

Leave a Reply