The electrochemical reduction of carbon dioxide (CO2) presents an innovative and sustainable way to mitigate climate change while generating valuable products. Researchers have long pursued methods to enhance product selectivity, often focusing primarily on the design and modification of catalysts used in the process. However, a new study highlights an often-overlooked aspect: the influence of electrolyte composition in controlling the reaction outcomes.
New Insights from Metal-Organic Frameworks
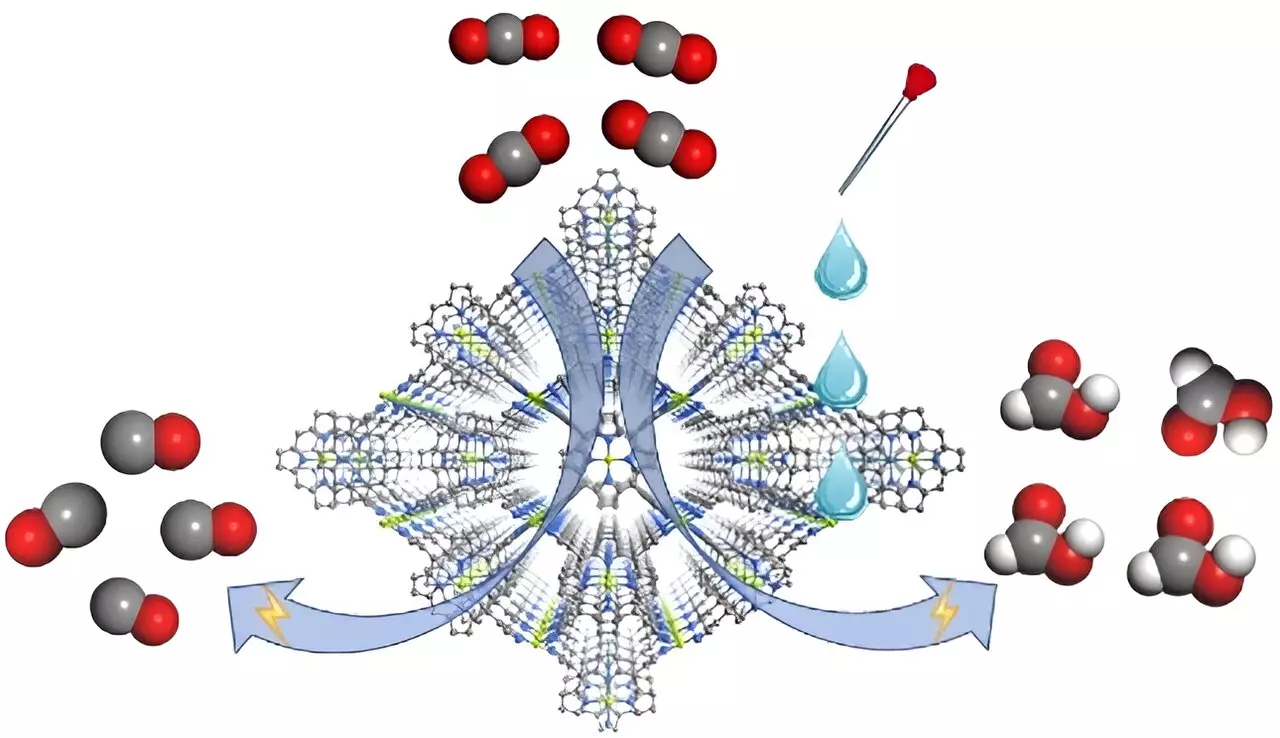
In a recent publication in Angewandte Chemie International Edition, a team of scientists, including prominent figures like Prof. Cao Rong and Prof. Zhang Teng, unveiled a remarkable metal-organic framework (MOF) electrocatalyst named FICN-8. This MOF is constructed with Cu(porphyrin)-derived ligands alongside Cu(pyrazolate) building units, forming a three-dimensional porous structure that allows for effective access to catalytic sites. This structural advantage confirms the significance of both effective electrocatalysts and optimized electrolyte configurations in achieving high selectivity for desired CO2 reduction products.
An outstanding feature of the FICN-8 MOF catalyst is its ability to exhibit varying product selectivity dependent on the electrolyte’s composition. In tests conducted with a tetrabutylammonium hexafluorophosphate (TBAPF6) and acetonitrile electrolyte, the catalyst achieved an impressive 95% selectivity for carbon monoxide (CO) production. Interestingly, the addition of water or trifluoroethanol (TFE) as a proton source drastically altered the major reduction product from CO to formic acid. Specifically, the researchers observed a peak Faradaic efficiency of 48% for formic acid when the electrolyte was enriched with specific concentrations of water or TFE.
To deepen their understanding of the underlying mechanisms that govern product selectivity, the researchers conducted a series of experiments focused on kinetic isotope effects (KIE). Their findings revealed a stark difference in the behavior of proton involvement during the reaction pathways of CO and formic acid production. While CO synthesis displayed a near-identical KIE value for hydrogen isotopes, the formic acid pathway exhibited a significantly larger KIE value of 3.7 ± 0.7, indicating clear proton participation. This could suggest that optimizing electrolyte composition not only influences selectivity but may also dictate the efficiency of specific pathways in CO2 reduction reactions.
This groundbreaking study sheds light on the crucial role of electrolyte composition in the electrochemical reduction of CO2. By demonstrating how the strategic tuning of electrolyte interactions can enhance the efficiency and selectivity of CO2 reduction products, it opens up avenues for developing new catalyst-electrolyte systems aimed at producing value-added chemicals. Future research may focus on expanding these concepts to create a more sustainable and efficient platform for CO2 recycling, which could have far-reaching implications in global efforts against climate change. Thus, as science progresses, it is paramount to consider not only catalyst design but also the broader electrolyte environment to harness the full potential of CO2 reduction technologies.

Leave a Reply