In the realm of biochemistry, few structures are as intriguing or as fundamental to molecular function as helices. Commonly observed in proteins and peptides, these twisted formations are not merely aesthetic; they play a vital role in how biomolecules operate within the biological landscape. The structural intricacies of helices are dictated by the specific arrangement of amino acids, the core building blocks of proteins. By analyzing these arrangements, researchers can uncover a wealth of information about the mechanisms that drive protein activity and stability.
The formation of helical structures hinges on the repetitive nature of amino acid sequences. Each sequence can undergo a transformation that introduces a chiral layer, leading to a unique spatial orientation that significantly impacts the characteristics of the peptide. Chirality, the property of a structure not being superimposable on its mirror image, is fundamental in biochemistry; it influences how enzymes interact with substrates and how receptors recognize biological signals. Thus, understanding chirality in the context of helices can shed light on larger biological processes.
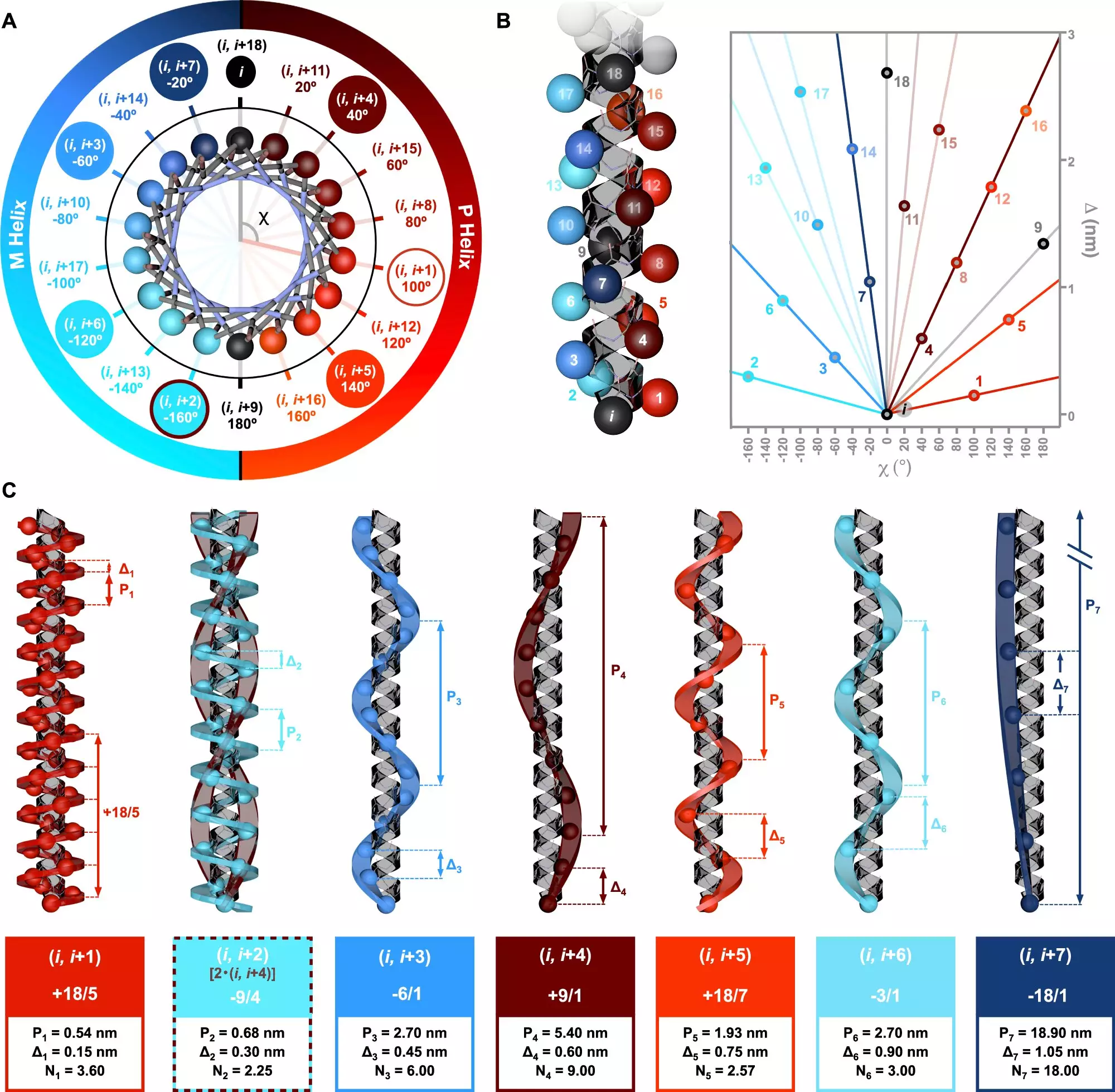
Recent research, led by Dr. Julián Bergueiro from the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), has delved into the complexities of chiral information in alpha-helical peptides. The groundbreaking study, published in Nature Communications, proposes a symmetry model that elucidates the interrelationships among various helical types. This model enhances the scientific community’s grasp of how sequences of amino acids govern the structural and functional properties of peptides.
Employing a combination of advanced computational methods and circularly polarized light spectroscopy, the research team was able to intricately dissect the molecular interactions at play. This innovative approach enabled them to validate theoretical predictions regarding exo-helical topologies.
The research findings have far-reaching implications, particularly in the fields of biotechnology and medicine. According to Dr. Bergueiro, variations in amino acid sequences lead to a plethora of helical configurations, which are crucial for understanding biological function. The ability to control the arrangement of monomers within polymer chains opens doors to tailored designs of new biomolecules, allowing for applications ranging from drug design to the creation of biocompatible materials.
This paradigm shift towards understanding and manipulating helical structures represents a new frontier in macromolecular engineering. By leveraging these insights, scientists can potentially discover new ways to create synthetic peptides that mimic or enhance natural biological effects.
The study of helical structures in peptides is not just a niche topic within biochemistry; it is a powerful lens through which we can explore fundamental principles of molecular biology. The groundbreaking research conducted by Dr. Bergueiro and his team signifies a pivotal progression in our comprehension of peptide chemistry. As we unlock the mysteries of these molecular formations, the potential for innovative applications in medicine and biotechnology becomes increasingly promising, paving the way for future discoveries that could revolutionize various fields.

Leave a Reply