Ovarian cancer, particularly high-grade serous ovarian carcinoma (HGSOC), stands as one of the most formidable adversaries in women’s health. The grim statistics surrounding its prognosis—a stark reality where the majority of patients succumb within five years of diagnosis—have triggered a dedicated quest for deeper understanding and earlier detection. Recent studies conducted on mice have cast a spotlight on potential sources of this aggressive cancer, focusing on the cells residing in the oviducts or uterine tubes. This article will delve into the groundbreaking findings suggesting that the origins of this malignancy might be traced back to these previously overlooked tissues, paving the way for innovative diagnostics and treatments.
Historically, ovarian cancer has been predominantly thought to originate in the ovaries themselves. Yet over the past decade, a paradigm shift has occurred, leading scientists to reevaluate these assumptions. Research has increasingly pointed towards the fallopian tubes as a critical site of origin for many ovarian tumors. In particular, lesions found at the distal ends of these tubes have shown genetic connections to tumors manifesting in the ovaries. Despite this promising lead, a comprehensive understanding of the specific cells involved in HGSOC genesis has remained elusive.
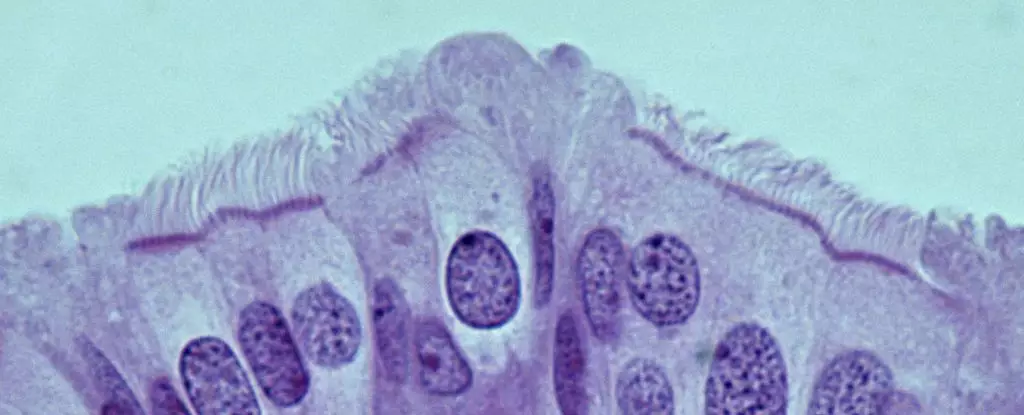
Recently, researchers led by Dr. Alexander Nikitin at Cornell University have made strides in identifying these vital cells. Their findings indicate that the most cancer-susceptible cells are not the stem cells previously identified in the ovaries, but rather pre-ciliated cells within the oviduct. This pivotal discovery suggests a reassessment of cancer-prone cell types, shifting the focus toward understanding their role in tumor formation.
In their findings, the team meticulously cataloged the various cell types within the mouse oviduct, marking a transformative moment in the quest for knowledge surrounding HGSOC. The research revealed that the pre-ciliated cells—transitional cells on their developmental journey to becoming fully ciliated—are notably prone to dysregulation when exposed to specific genetic mutations associated with HGSOC. This innovative approach not only uncovers the cellular landscape of the oviduct but also emphasizes the significance of understanding how these cells initiate cancerous transformations.
Nikitin’s groundbreaking work challenges the existing perception of ovarian cancer’s cell lineage and sheds light on the intricate mechanisms that may drive disease initiation. By identifying these pre-ciliated cells as a potential origin point, the research team opens new discussion pathways toward targeted diagnostic markers and streamlined therapeutic interventions.
The Genetic Connection and Ciliogenesis
At the crux of this research lies the identification of two genetic mutations tied to the development of HGSOC, which appear to disrupt the usual processes within pre-ciliated cells. Rather than functioning normally, these mutations propel the cells towards malignant growth, leading to effective cancer formation. This underscores a fascinating link between the regulation of cilia formation—an essential function of these cells—and ovarian cancer susceptibility. Notably, similar issues in cilia development have been linked with pancreatic cancer, suggesting a broader implication of ciliogenesis in malignant transformations.
This connection is emblematic of the complex interactions between genetic predisposition and cellular health. By exploring these mechanisms, scientists aim to unravel the pathways that facilitate tumor growth, providing a foundation for future therapeutic approaches targeting these disruptions.
Implications for Early Detection and Treatment
The ramifications of these discoveries could be monumental. If the mechanisms identified in the mouse model are corroborated in humans, we stand on the cusp of significant advancements in the early detection of HGSOC. Given that about 80 percent of cases are diagnosed at advanced stages, a reliable diagnostic method built upon these findings could revolutionize patient outcomes, potentially increasing survival rates significantly.
While the current study presents foundational knowledge, it also advocates for further exploration into other genetic mutations related to HGSOC. Understanding the disparate impacts of these mutations on pre-ciliated cells versus stem cells could illuminate new avenues for research, ultimately translating into clinical applications that better serve women’s health.
The exploration of ovarian cancer’s origins through the lens of mouse studies has illuminated previously hidden aspects of disease initiation. By pinpointing the role of pre-ciliated cells in the oviduct in the development of HGSOC, researchers have not only challenged long-held beliefs but have also set the stage for a new era in diagnostic and treatment strategies. Ongoing research will be essential in verifying these findings in human biology, but the potential for life-saving advancements in ovarian cancer care is now more tangible than ever.

Leave a Reply