In cutting-edge research conducted at the Oak Ridge National Laboratory, scientists have illuminated pivotal insights regarding an enzyme’s role in cancer proliferation. This breakthrough relates specifically to serine hydroxymethyltransferase (SHMT), an enzyme integral to cellular division and metabolism. Previous understanding of SHMT’s catalytic mechanisms had persisted in debate for decades, but through innovative neutron experiments, researchers have unveiled the enzyme’s atomic-level functionality. These revelations not only enhance our grasp of cancer biology but also pave the way for developing novel inhibitors that could effectively thwart aggressive cancer growth.
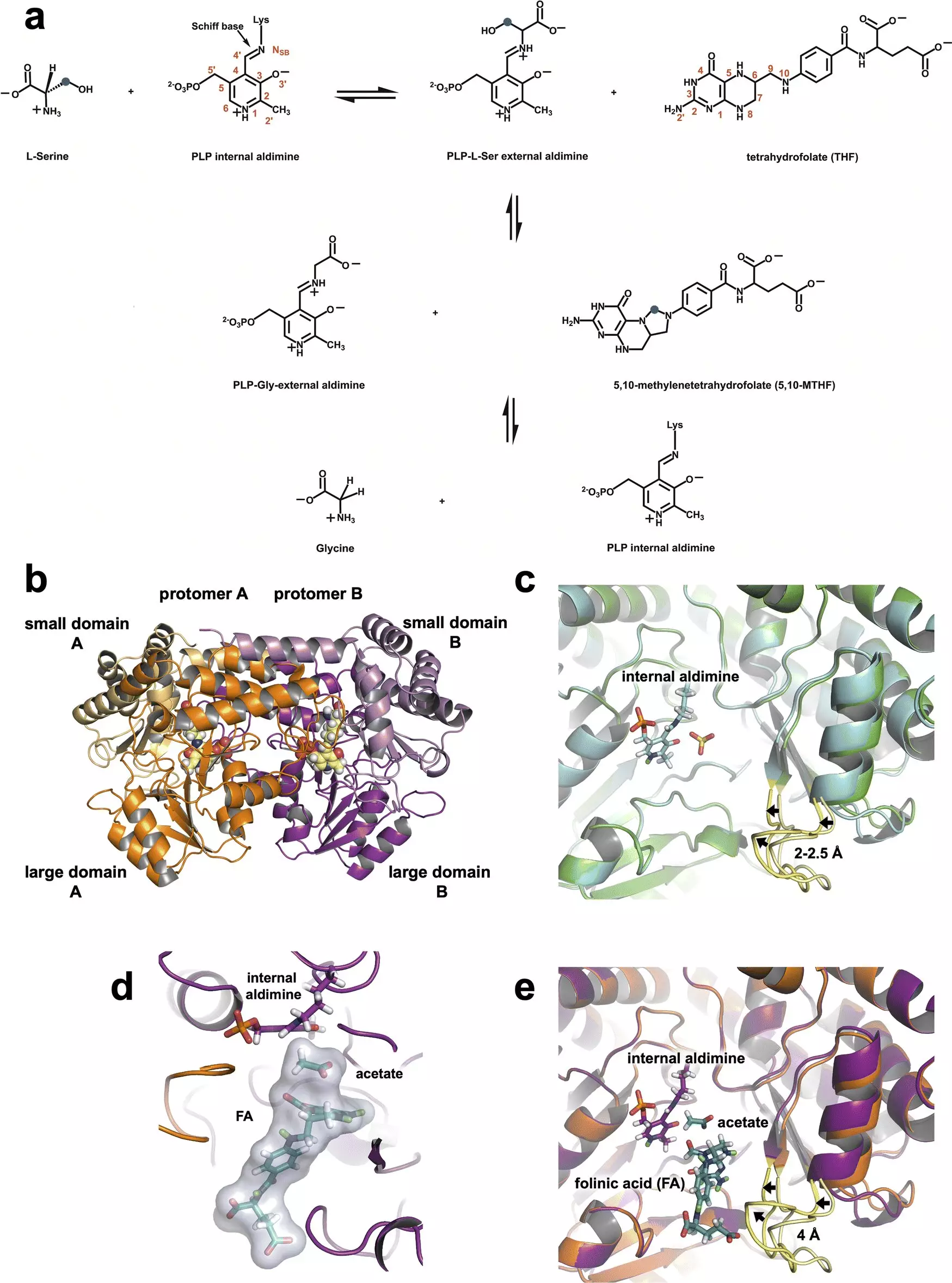
SHMT plays a critical role in one-carbon metabolism, a biochemical pathway essential for producing nucleic acids necessary for DNA and RNA synthesis. By converting serine into glycine, the enzyme facilitates critical metabolic processes within the mitochondria, the energy-producing organelles of cells. In their recent experiments, the researchers meticulously tracked the interactions of a single glutamate residue within SHMT’s active site, which surprisingly retained a proton that facilitates its dual function as both an acid and a base. Understanding this proton transfer capacity is a crucial leap forward for drug design, as it highlights a specific point where enzymatic activity can potentially be inhibited.
Utilizing advanced neutron scattering techniques, the research team gained unprecedented visibility into the dynamics of hydrogen atoms within the enzyme. Neutrons, with their ability to penetrate light elements like hydrogen, provided vital data on the precise locations of hydrogen atoms in SHMT. This was made possible through the facilities at the Spallation Neutron Source and the High Flux Isotope Reactor, which enabled the team to gather meaningful information regarding the electrostatic environment surrounding the enzyme. As stated by biochemist Victoria Drago, the importance of understanding hydrogen positioning cannot be overstated, as hydrogen atoms often dictate the reactivity of enzymes.
The implications of these findings are profound. As cancers exploit metabolic pathways to fuel their relentless progression, targeting enzymes like SHMT presents a crucial opportunity to intervene earlier in the cancerous degeneration process. While existing treatments often target downstream processes, an inhibitor designed to interfere with SHMT’s activity could potentially halt cancer cell reproduction before it accelerates. This approach, unlocking the inherent metabolic processes within cancer cells, fundamentally transforms the paradigm of drug development, emphasizing the need for targeted therapies.
Yet, the journey toward effective cancer treatments remains fraught with challenges. Unlike treatments for infectious diseases that target foreign pathogens, cancer therapies must grapple with the delicate balance of targeting malignant cells without harming healthy tissue. Cancer cells can adapt to bypass inhibited pathways, underscoring the need for innovative strategies and combination therapies to preemptively thwart their evasive nature. Identifying the specific roles of enzymes within the cancer metabolic framework is imperative to circumvent drug resistance.
Looking towards the future, the intersection of artificial intelligence (AI) and biochemical research promises to revolutionize treatment strategies. Visionary comments from William Nelson of Johns Hopkins highlight an emerging landscape where genomic sequencing could delineate personalized drug formulations at an unprecedented speed. Nonetheless, while such technological advancements are on the horizon, researchers acknowledge that achieving this level of sophistication is still a work in progress.
The insights gained from neutron experiments not only elucidate the complexities of SHMT but also set the stage for targeted inhibitor development in cancer therapy. By disrupting the essential functions of enzymes like SHMT, scientists anticipate a significant shift in therapeutic paradigms that prioritize precision medicine. As research continues to uncover the intricate details of metabolic pathways, the dream of curbing cancer’s rapid expansion may soon shift from aspiration to reality. This groundbreaking work is a testament to the critical need for ongoing inquiry and technological advancement in the fight against one of humanity’s most daunting health challenges.

Leave a Reply