In the intricate world of molecular interactions, the process of electron movement can be likened to a delicate dance, one that occurs at unimaginably swift rates. At the heart of this phenomenon are two pivotal processes: photosynthesis in nature and the function of solar panels, which harness sunlight to produce energy. Through the mechanisms of photosynthesis, plants and bacteria convert light energy into chemical energy, while solar panels utilize photovoltaics to achieve the same end, albeit in a different medium. These processes are intrinsically linked by the underlying principles of charge transfer and electronic movement at a molecular scale.
When molecules absorb light, they experience a redistribution of electronic density, a phenomenon that unfolds at an ultrafast pace. Understanding this electronic dance—how electrons and nuclei interact and transfer charge—has profound implications for both fundamental chemistry and the development of advanced technologies. Capturing the essence of these dynamics requires tools capable of probing their rapid occurrence on exceptionally short timescales.
Traditionally, the study of electron and charge transfer has faced significant temporal resolution challenges. Conventional techniques have made it difficult to observe the initial moments following photoionization, a critical phase where the charge migration begins. However, groundbreaking advancements in ultrafast spectroscopy have revolutionized our ability to measure these fleeting dynamics. Employing attosecond extreme-ultraviolet pulses derived from high-order harmonic generation and free electron lasers allows researchers to observe events on timescales ranging from femtoseconds (10^-15 seconds) to attoseconds (10^-18 seconds).
Despite these technological advancements, a comprehensive understanding of the initial stages of charge transfer after photoionization remains elusive. This gap in knowledge has motivated a collaborative effort among researchers from esteemed institutions, including Politecnico di Milano and the Madrid Institute for Advanced Studies in Nanoscience. Their recent work, published in *Nature Chemistry*, heralds a significant breakthrough in elucidating the ultrafast dynamics of molecular systems.
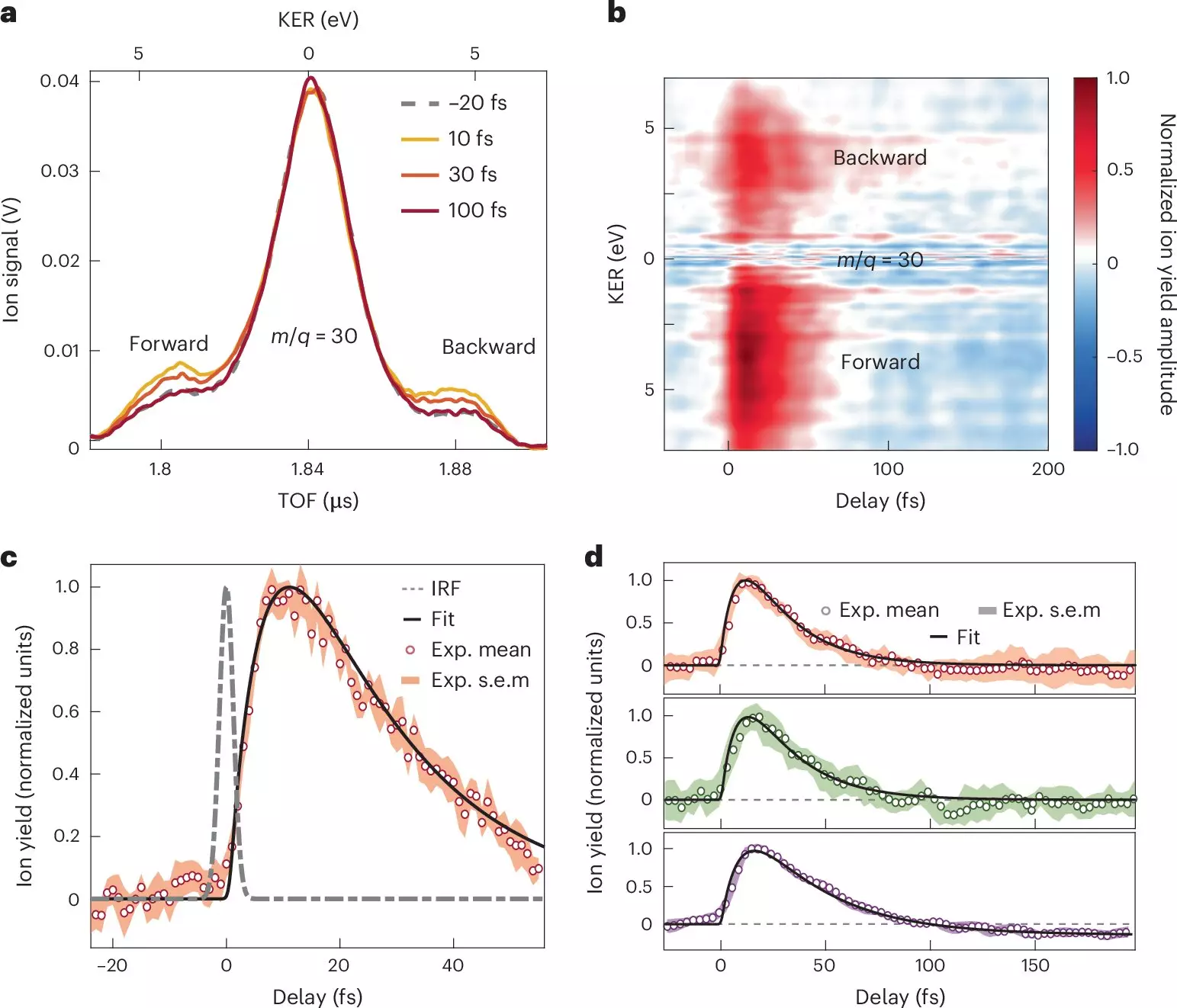
In their study, the researchers focused on nitroaniline molecules, utilizing attosecond pulses to investigate the earliest phases of charge transfer. By adopting an innovative approach that combines attosecond extreme-ultraviolet-pump and few-femtoseconds infrared-probe spectroscopy, alongside sophisticated many-body quantum chemistry calculations, the team has been able to detail the rapid processes that underpin charge transfer.
One of the most striking revelations of the study is the astonishing speed at which electron transfer from the amino donor group occurs—within less than 10 femtoseconds. This rapid transfer is not a mere isolated event; it is driven by a synchronized movement of both nuclei and electrons, highlighting the intricate coupling between these two aspects of molecular behavior. Following this prompt transfer is a relaxation process that unfolds over a sub-30-femtosecond timescale, as nuclear wave packets evolve within the excited electronic states of the molecular cation.
These findings hold significant implications for the broader understanding of electron-nuclear coupling and its effect on electron donor-acceptor systems. By mapping the timeframes required for charge migration from the donor unit to adjacent chemical bonds, this research sheds light on the intricate structural changes that accompany charge transfer processes. The authors suggest that their work not only clarifies existing theoretical frameworks but also challenges and refines the conventional diagrams used to qualitatively predict charge migrations in organic molecules.
The insights gleaned from this study pave the way for future inquiries into the realm of attosecond science. Researchers are now better equipped to investigate the complex interplay of electronic states and molecular dynamics, thus setting the stage for innovations that could transform our understanding of chemical reactions and catalysis.
The exploration of ultrafast electron dynamics provides a gateway to deeper insights into molecular interactions and processes that govern life and technology. By examining the precision of atomic interactions on unprecedented timescales, scientists can refine their theoretical models and, crucially, enhance practical applications across various fields such as materials science, photovoltaics, and photochemistry. As we continue to unravel the complexities of these rapid phenomena, the potential for transformative advancements becomes ever clearer, underscoring the vital fusion of theoretical understanding and experimental precision in modern science.

Leave a Reply