Proteins are essential macromolecules that play critical roles in the functioning of all living organisms. Their ability to participate in a multitude of cellular processes hinges on their intricate structures. A core tenet in biochemistry is that the architecture of a protein directly dictates its functionality. When this structure is compromised, even slightly, the implications can be severe, potentially leading to debilitating diseases. In this context, the protein myo-inositol-1-phosphate synthase (MIPS) emerges as an intriguing subject for study, demonstrating a remarkable capacity to alter its conformation. This unique adaptability underlines not only its physiological importance but also propels research forward in understanding protein dynamics.
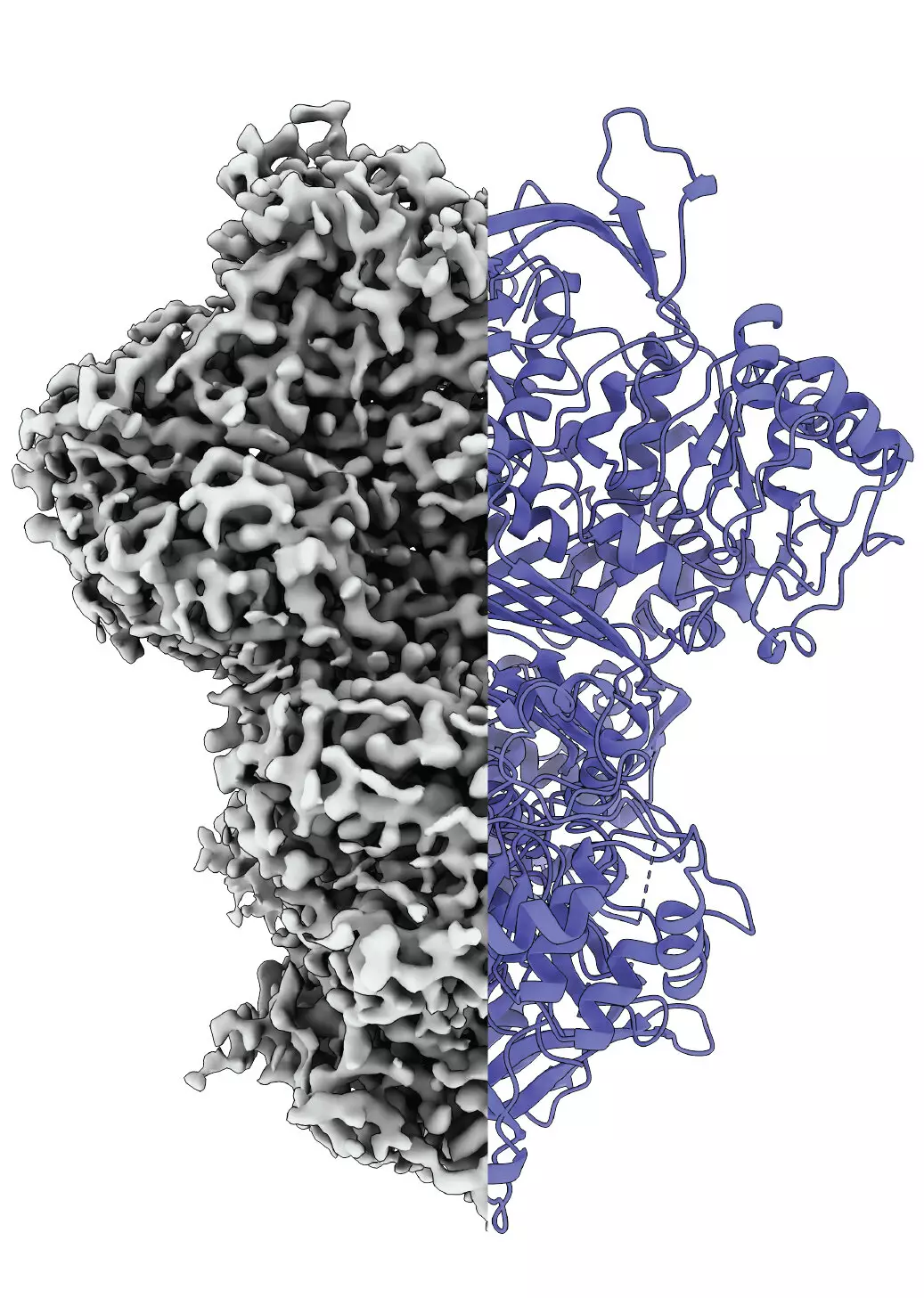
Recent groundbreaking research conducted collaboratively by scientists from Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center has illuminated the structural transitions occurring within MIPS. Throughout their investigation, detailed in the Proceedings of the National Academy of Sciences, the researchers harnessed cutting-edge techniques such as cryo-electron microscopy to observe MIPS in action. This method allowed the team to visualize the protein’s reorganization in real-time under conditions that closely mimic its natural environment—a factor often overlooked in traditional analyses.
Their results unveiled that MIPS fluctuates through at least three distinct conformational states during its functional cycle: a disordered state, an ordered state, and a previously unidentified intermediate state. Although the precise role of this intermediate state remains speculative, it may be crucial in facilitating the protein’s interaction with other molecules, potentially enhancing catalytic efficiency or stabilizing hydration dynamics essential for biochemical reactions.
Implications for Inositol Production
MIPS is integral to the biosynthesis of inositol, a compound revered for its involvement in various cellular functions. While commonly referred to as vitamin B8, it is technically not classified as a true vitamin because the human body can synthesize it. Still, the significance of inositol in vital processes like cell signaling, neuronal function, and lipid metabolism can hardly be overstated. Toni Träger, a researcher involved in the study, emphasized that MIPS is a pivotal component in a complex metabolic pathway leading to inositol production. This underscores the necessity of understanding MIPS’s structure and function, especially within the context of metabolic health.
The investigation did not stop with MIPS. The research team delved deeper, assessing other proteins within the isomerase family to discern whether they exhibit similar structural dynamics. This venture involved analyzing data on over 340 isomerases, resulting in compelling evidence suggesting that many share the same conformational variability as MIPS. This revelation opens new avenues in protein research, providing an expanded framework for understanding the diverse functionality inherent to this class of proteins.
The ramifications of these findings extend far beyond basic scientific inquiry. Gaining deeper insights into the nuances of metabolic pathways linked to MIPS paves the way for potential therapeutic advancements. By understanding the molecular intricacies governing proteins like MIPS, researchers could unlock new opportunities for drug development and treatment strategies aimed at metabolic disorders. As Professor Panagiotis Kastritis suggests, this work is merely a foundational step towards a more comprehensive grasp of protein behavior and its wider implications for health and disease.
The study of MIPS and its functional dynamics marks a significant milestone in our understanding of protein behavior. With its ability to transition among various structural states, MIPS does not only help in elucidating the intricacies of protein function but also serves as a potential model for future investigations into related proteins. As researchers continue to unravel these molecular phenomena, it is imperative to recognize the interconnectedness of protein structure, function, and the broader implications for human health. The journey ahead will likely unveil even more profound insights, reaffirming the critical role of proteins in life’s intricate tapestry.

Leave a Reply