Scientists from The Hebrew University of Jerusalem, led by Profs. Daniel Strasser and Roi Baer, have recently made a groundbreaking discovery in molecular physics. Their research has uncovered unexpected symmetry-breaking dynamics in ionized carbon dioxide dimers, shedding light on structural changes under extreme ultraviolet (EUV) radiation. This discovery has significant implications for atmospheric and astrochemistry, offering a deeper understanding of molecular behavior in challenging environments.
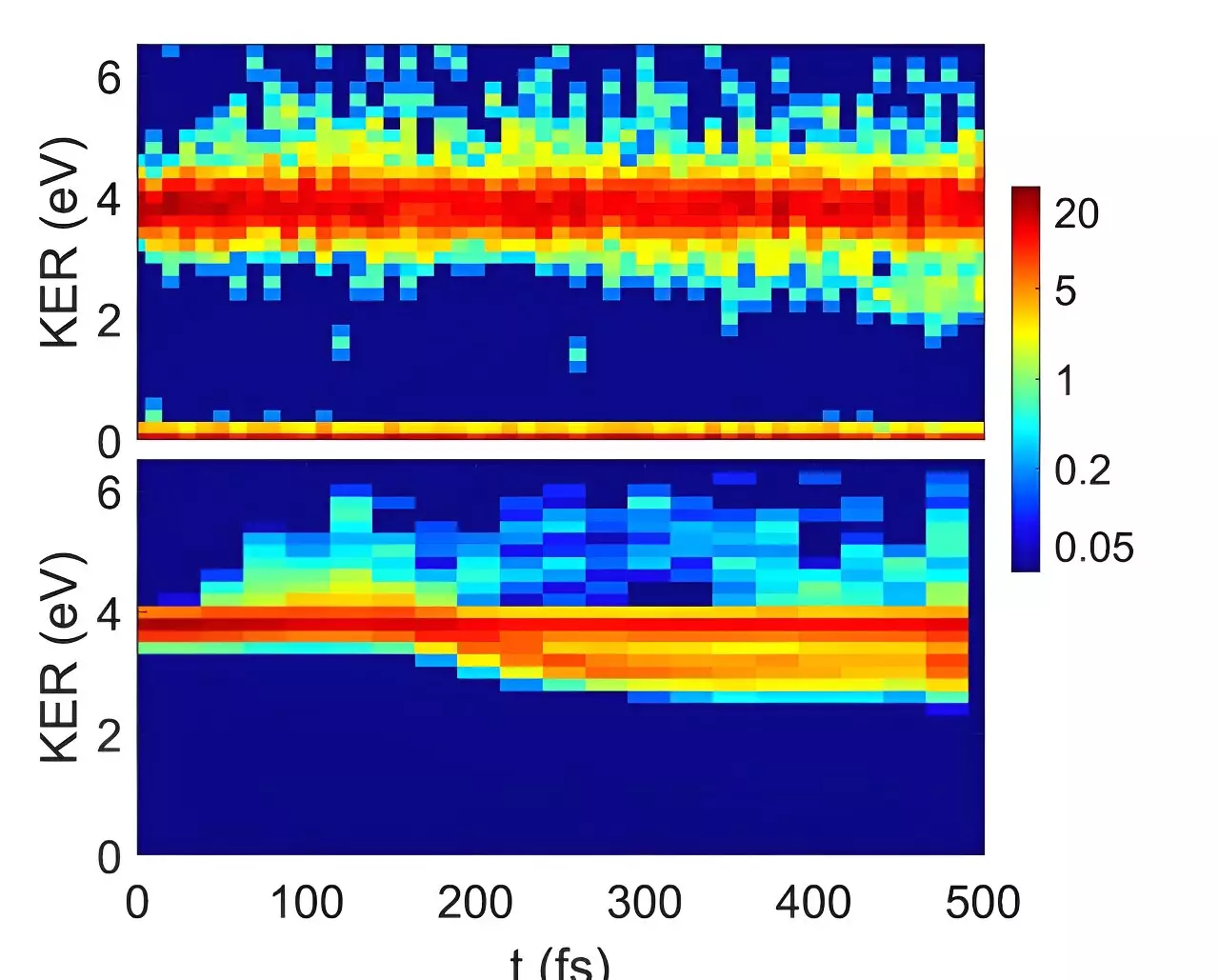
The research paper titled “Symmetry-breaking dynamics of a photoionized carbon dioxide dimer” has been published in Nature Communications. The international collaboration between researchers from Israel, Germany, and the FLASH free electron laser facility at DESY has demonstrated that ionized CO2 dimers undergo asymmetric structural rearrangements, leading to the formation of CO3 moieties. Contrary to expectations based on quantum mechanics, the ionized dimers exhibit a phenomenon called symmetry-breaking, where one of the molecules rotates around its axis and points towards its partner within 150 femtoseconds.
The team utilized ultrafast EUV pulses from the FLASH free electron laser to confirm the second quantum chemistry model’s prediction of symmetry-breaking in ionized carbon dioxide dimers. This discovery challenges traditional quantum mechanics theories and raises questions about how symmetry-breaking can occur in molecular clusters. The researchers explain that the molecules exist in a superposition of two symmetry-breaking states, which collapse upon measurement, resulting in the observed structural rearrangements.
Prof. Strasser, the lead author of the study, emphasized the importance of combining experimental techniques with theoretical modeling to uncover unexpected molecular behavior. The insights gained from this research could potentially open new avenues for carbon dioxide chemistry and enhance our understanding of planetary and atmospheric processes. Prof. Baer, who led the theoretical modeling, highlighted the importance of comparing theory with experimental measurements to improve predictions of chemical reactions in remote environments.
The study’s results have broad implications for atmospheric chemistry and astrochemistry. The discovery of asymmetric structural rearrangements and the formation of CO3 moieties provide a deeper understanding of molecular processes under extreme conditions. The time-resolved dynamics observed in ionized carbon dioxide dimers offer new insights into atmospheric carbon dioxide cycles and chemical evolution in cold outer space environments.
This research was made possible through international collaboration and the utilization of state-of-the-art facilities like the FLASH2 free electron laser at DESY. The team’s innovative approach sets the stage for further investigations into the behavior of molecular clusters under extreme conditions. The findings from this study could have applications in atmospheric science, astrochemistry, and novel chemical synthesis methods. Overall, this research marks a significant advancement in molecular physics and opens up new possibilities for studying molecular behavior in challenging environments.

Leave a Reply